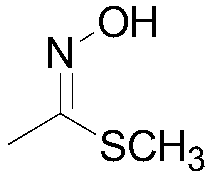
Preparation method of methomyl oxime
A technology of methomyl oxime and acetaldehyde oxime, applied in oxime preparation, organic chemistry, etc., can solve the problems of difficult precise control of reaction, many impurities produced, easy decomposition of chlorinated acetaldehyde oxime, etc., and achieve material dispersibility and flow Enhanced performance, avoiding too much or too little, high quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of methomyl oxime of the present invention, comprises the following steps:
[0039] (1) In a reactor with a stirrer, dissolve 29.5g (0.5mol) of acetaldehyde oxime in 100g of dichloroethane, stir evenly to obtain an acetaldehyde oxime organic solution, cool to 5°C, and store ; Dissolve 35.5g (0.5mol) of chlorine in 149.7g of dry ethylene dichloride, stir evenly to obtain an organic chlorine solution, cool to 5°C, and store; 35g (0.5mol) of sodium methyl mercaptide and Dissolve 20 g (0.5 mol) of sodium hydroxide in 150 g of dichloroethane, stir evenly to obtain an organic mixed solution of sodium methyl mercaptide and sodium hydroxide, cool to 5° C., and store.
[0040] (2) Turn on the microreactor (model LFR15-328-1A), cool down to 5°C, and at the same time, prepare 129.5g of acetaldehyde oxime organic solution and 185.2g of chlorine organic solution at a rate of 10g / min and 14.3g / min respectively Pump into the microreactor and carry out the chlorin...
Embodiment 2
[0044] A preparation method of methomyl oxime of the present invention, comprises the following steps:
[0045] (1) In a reactor with a stirrer, dissolve 29.5g (0.5mol) of acetaldehyde oxime in 100g of 2-picoline, stir evenly to obtain an acetaldehyde oxime organic solution, cool to 5°C, Preserve; similarly dissolve 35.5g (0.5mol) of chlorine in 149.7g of dry 2-picoline, stir well to obtain an organic solution of chlorine, cool to 5°C, and store; 35g (0.5mol) of methyl mercaptan Sodium and 20 g (0.5 mol) of sodium hydroxide were dissolved in 150 g of 2-picoline, stirred evenly to obtain an organic mixed solution of sodium methyl mercaptide and sodium hydroxide, cooled to 5° C., and stored.
[0046] (2) Turn on the microreactor (model LFR15-328-1A), cool down to 5°C, and at the same time, prepare 129.5g of acetaldehyde oxime organic solution and 185.2g of chlorine organic solution at a rate of 10g / min and 14.3g / min respectively Pump into the microreactor and carry out the chlo...
Embodiment 3
[0050] A preparation method of methomyl oxime of the present invention, comprises the following steps:
[0051] (1) In a reactor with a stirrer, dissolve 29.5g (0.5mol) of acetaldehyde oxime in 100g of dimethyl sulfoxide, stir evenly to obtain an acetaldehyde oxime organic solution, cool to 5°C, Preserve; Dissolve 35.5g (0.5mol) of chlorine in 149.7g of dry dimethyl sulfoxide, stir evenly to obtain an organic solution of chlorine, cool to 5°C, and store; 35g (0.5mol) of methyl mercaptan Sodium and 20 g (0.5 mol) of sodium hydroxide were dissolved in 150 g of dimethyl sulfoxide, stirred evenly to obtain an organic mixed solution of sodium methyl mercaptide and sodium hydroxide, cooled to 5° C., and stored.
[0052] (2) Turn on the microreactor (model LFR15-328-1A), cool down to 5°C, and at the same time, prepare 129.5g of acetaldehyde oxime organic solution and 185.2g of chlorine organic solution at a rate of 10g / min and 14.3g / min respectively Pump into the microreactor and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com