Drug for promoting recovery from chronic spinal cord injury and preparation method and application thereof
A spinal cord injury and drug technology, applied in the field of biomedicine, to achieve the effect of promoting neuron protection and axon regeneration, improving functional recovery, and inhibiting inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 experimental material and method
[0032] 1. Experimental materials:
[0033]1.1 Drug: RGFP966 (provided by Shanghai Yijie Biotechnology Co., Ltd.)
[0034] 1.2 Animals Wild-type mice (C57BL6, provided by the Animal Experiment Middle School of Xi'an Jiaotong University School of Medicine)
[0035] 2. Experimental method
[0036] 2.1 Spinal cord injury model preparation
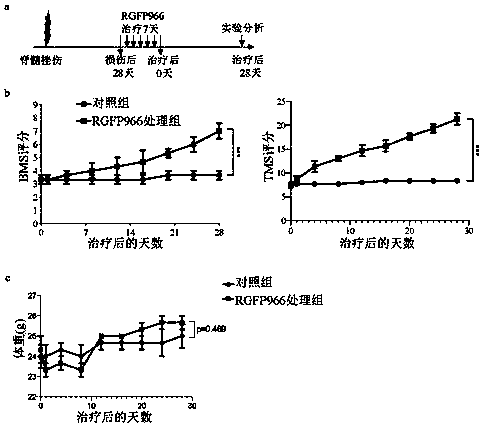
[0037] Spinal cord contusion model: 4-6 week old wild-type mice (C57BL6, provided by the Animal Experiment Middle School of Xi'an Jiaotong University School of Medicine) were randomly selected, weighed, and anesthetized by isoflurane inhalation. After successful anesthesia, cut off the hair in the operation area, take the prone position, and use iodophor and alcohol to disinfect the operation area. Make a central incision on the back, incision layer by layer, bluntly dissect the paravertebral musculature and bite off T8-9. After fully exposing the T8-9 lamina, gently resect the entire s...
Embodiment 2
[0044] Embodiment 2 Drug Usage and Dosage
[0045] Usage one:
[0046] 1. Drug preparation:
[0047] (1) 100mg RGFP966 was dissolved in 2mL DMSO to prepare a storage solution with a concentration of 50mg / mL
[0048] (2) Configure the carrier solvent, dissolve 30% hydroxypropyl-β-cyclodextrin (2-Hydroxypropyl-β-cyclodextrin) in 100mM sodium acetate (Sodium acetate), adjust the pH to 5.6; the carrier solvent can also be Mix DMSO, PEG300, Tween, and normal saline in sequence, and the volume ratio of the final solution is 5% DMSO, 40% PEG300, 10% Tween80, and 45% normal saline; prepare and use immediately;
[0049] (3) 1 part of storage solution and 49 parts of carrier solution were mixed to obtain an injection solution, and the concentration of RGFP966 was respectively 1 mg / mL.
[0050] 2. Drug injection:
[0051] (1) The RGFP966 treatment group was given an injection solution of 10mL / kg each time, subcutaneously (that is, the dosage of RGFP966 was 10mg / kg)
[0052] (2) The ...
Embodiment 3
[0069] Example 3 High expression of HDAC3 in macrophage / microglia after acute and chronic spinal cord injury
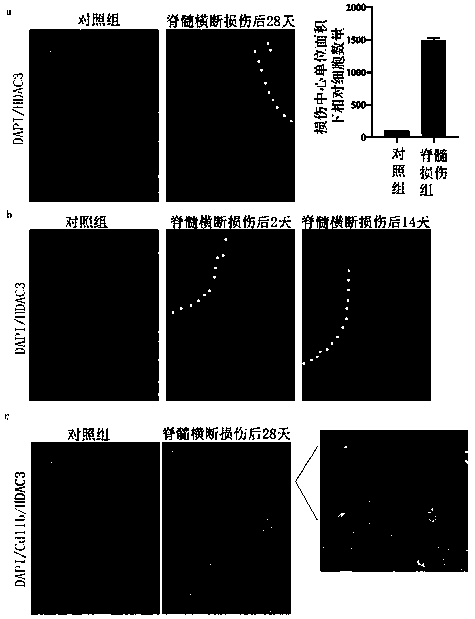
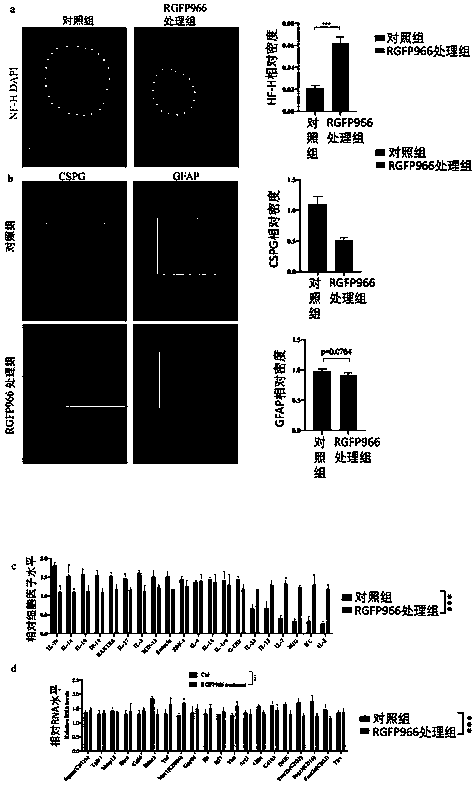
[0070] Firstly, the dynamic expression of HDAC3 was detected by immunohistochemistry at 28 days after spinal cord injury (28dpi). In an in vivo model of dorsal tract transection of spinal cord injury, a marked high expression of HDAC3 was found in the center of the lesion 28 days after injury ( figure 1 a). Temporal analysis showed that HDAC3 upregulation was detectable at 2dpi, peaked at 14dpi, and continued until 28dpi with a slight attenuation ( figure 1 b). In chronic spinal cord injury, HDAC3 was markedly upregulated in the nuclei of CD11b-positive cells when overlaid with CD11b, a cell surface marker of microglia- and monocyte-derived inflammatory macrophages in the central nervous system ( figure 1 c). Thus, different class I HDACs appear to be differentially regulated in different cell types after SCI. Co-immunostaining for HDAC3 and IBA1 confirmed the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com