Pharmaceutical composition of 2-aminopyrimidine compound
A composition and compound technology, which can be used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as weak inhibitory activity of wild-type EGFR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
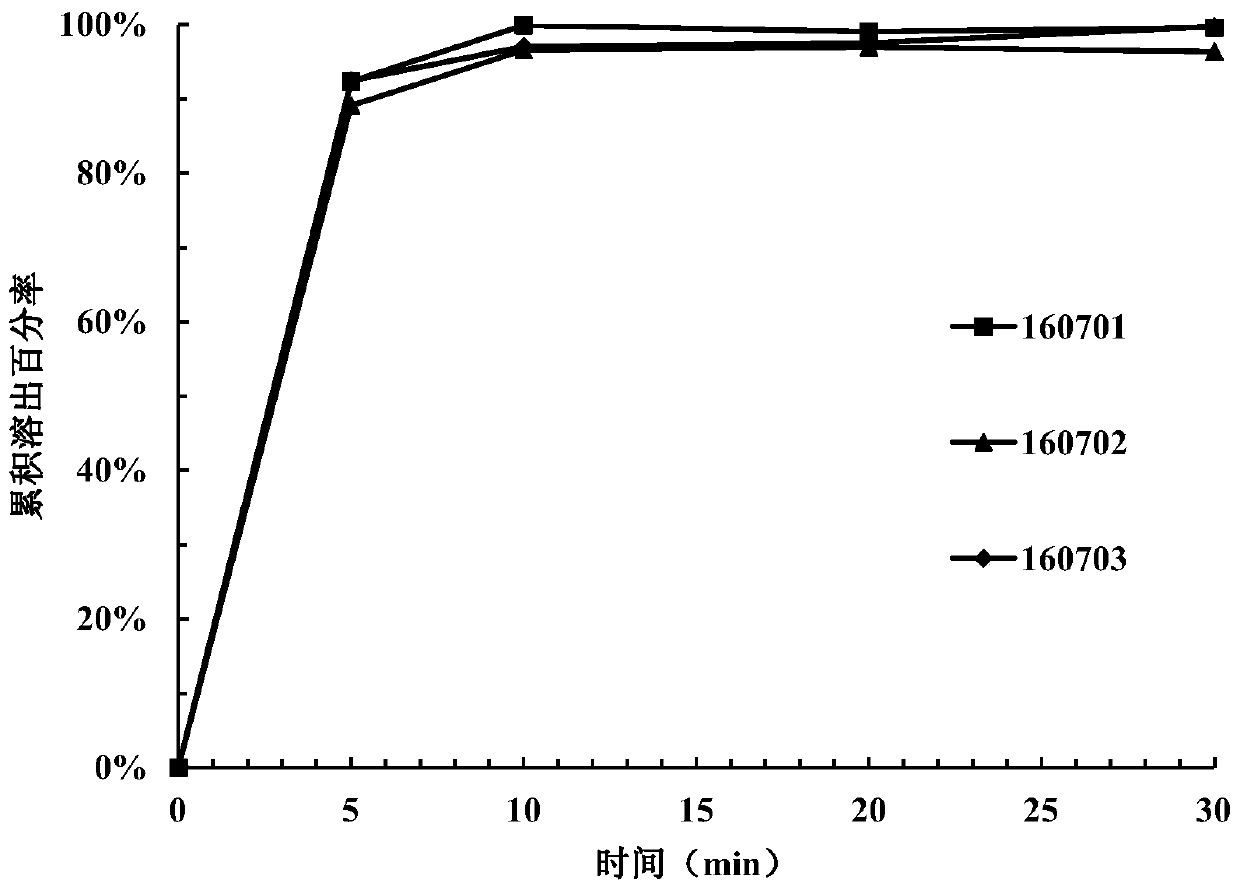
Embodiment 1
[0132] components Proportion / % Content of 1 tablet / mg Compound of formula I 25 80 lactose 16.75 53.60 microcrystalline cellulose 50.25 160.80 Crospovidone 3.5 11.20 Sodium stearyl fumarate 3 9.60 colloidal silica 1.5 4.80
[0133] Weighing: Accurately weigh the compound of formula I, lactose, microcrystalline cellulose (PH302), crospovidone, sodium stearyl fumarate (S96), and colloidal silicon dioxide according to the scale of 500 tablets.
[0134] Mixing: Premix other materials except sodium stearyl fumarate first, then sieve through a 40-mesh stainless steel sieve for 3 times, add sodium stearyl fumarate to the sieved material and mix well, take 9 samples at different positions , to measure content uniformity.
[0135] Tablet compression: Calculate the standard tablet weight according to the measurement results of the intermediate content, control the tablet weight ± 3%, hardness 90-120N, and compress the tablet.
...
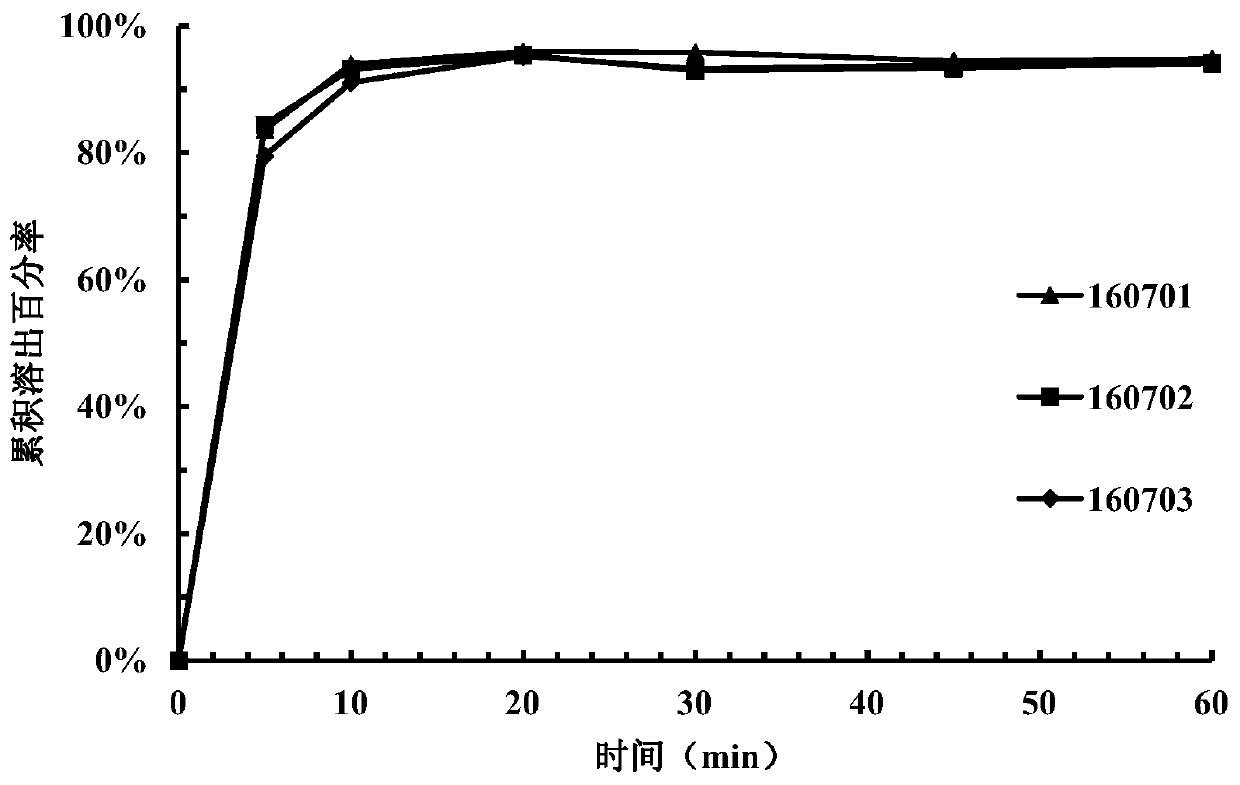
Embodiment 2
[0138] components Proportion / % Content of 1 tablet / mg Compound of formula I 25 80 Mannitol 50.25 160.80 microcrystalline cellulose 16.75 53.60 Crospovidone 3.5 11.20 Sodium stearyl fumarate 3 9.60 colloidal silica 1.5 4.80
[0139] Weighing: Accurately weigh the compound of formula I, mannitol, microcrystalline cellulose (PH302), crospovidone, sodium stearyl fumarate (S96), and colloidal silicon dioxide according to the scale of 500 tablets.
[0140] Mixing: Premix other materials except sodium stearyl fumarate, and then pass through a 40-mesh stainless steel sieve for 3 times, add sodium stearyl fumarate to the sieved materials and mix evenly, and measure the content of intermediates.
[0141] Tablet compression: Calculate the standard tablet weight according to the measurement results of the intermediate content, control the tablet weight ± 3%, hardness 90-120N, and compress the tablet.
[0142] Coating: The concent...
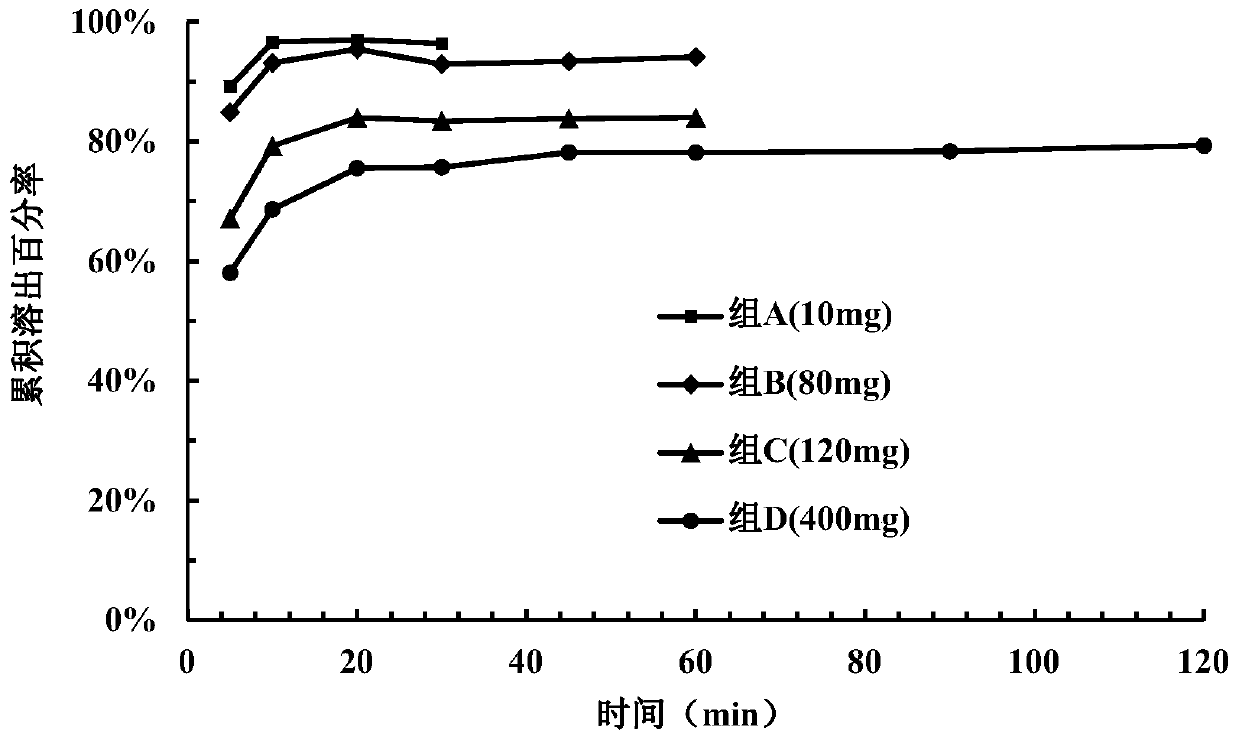
Embodiment 3~ Embodiment 6
[0144]
[0145] Weighing: Accurately weigh the compound of formula I, lactose, microcrystalline cellulose (PH302), crospovidone, and sodium stearyl fumarate (S96) according to the scale of 1100 tablets.
[0146] Mixing: Premix other materials except sodium stearyl fumarate, and then pass through a 40-mesh stainless steel sieve for 3 times, add sodium stearyl fumarate to the sieved materials and mix evenly, and measure the content of intermediates.
[0147] Tablet compression: Calculate the standard tablet weight according to the measurement results of the intermediate content, control the tablet weight ± 3%, hardness 90-120N, and compress the tablet.
[0148] Coating: The concentration of the coating solution is prepared to be 12%, and the weight gain of the coating is 3-4%.
[0149] Take 15 tablets from the tablets prepared in each example and divide them into 3 parts for dissolution investigation (dissolution medium pH=3.8). Samples were taken and measured at 5 minutes, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com