Hindered phenol antioxidant and preparation method thereof
A technology of hindered phenols and antioxidants, applied in the field of hindered phenols antioxidants and its preparation, can solve the problems of polymer antioxidants, the inability to use antioxidant substances, and the impact of antioxidant effects, etc., to achieve improved Heat stability and anti-oxidation performance, improvement of heat resistance stability, effect of enhancing anti-oxidation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing the hindered phenolic antioxidant described in the above technical scheme, including the following steps:
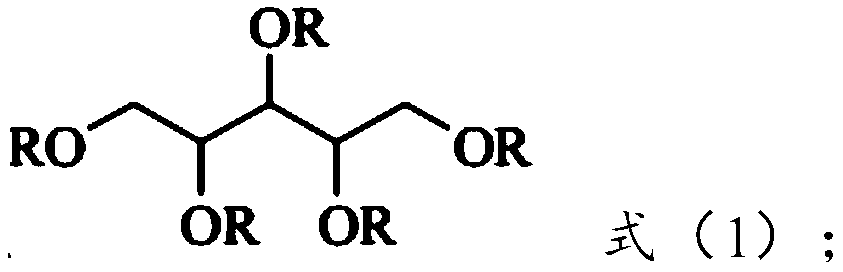
[0037] Under the action of a catalyst, β-(3,5-di-tert-butyl-4-hydroxyphenyl) methyl acrylate is esterified with xylitol to form the hindered phenolic antioxidant represented by formula (1) .
[0038] The reaction route is as follows:
[0039]
[0040] In the present invention, the source of the methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylate is not particularly limited, and is generally commercially available or prepared according to a preparation method well known to those skilled in the art That's it.
[0041] In the present invention, a specific xylitol is used to react with β-(3,5-di-tert-butyl-4-hydroxyphenyl) methyl acrylate to prepare an antioxidant. Compared with other alcohols, xylitol is used with the above The product made by acrylate can have more suitable molecular weight, melting point, good c...
Embodiment 1
[0058] 1.1 Preparation
[0059] Add 84.8g (0.29mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl)methyl acrylate into a 250mL four-necked flask equipped with an electric stirrer, thermometer, condenser and methanol receiver. , Xylitol 7.6g (0.05mol), catalyst sodium methoxide 0.10g, heating and stirring, control the temperature at 180±5℃, first react at normal pressure for 6h, then react under reduced pressure for 2h, extract the methanol gas generated by the reaction, and react to No more methanol is formed. The temperature is lowered, the product is recrystallized with isopropanol, filtered, washed with isopropanol, and dried to obtain the product, which has a white solid appearance.
[0060] 1.2 Characterization and testing
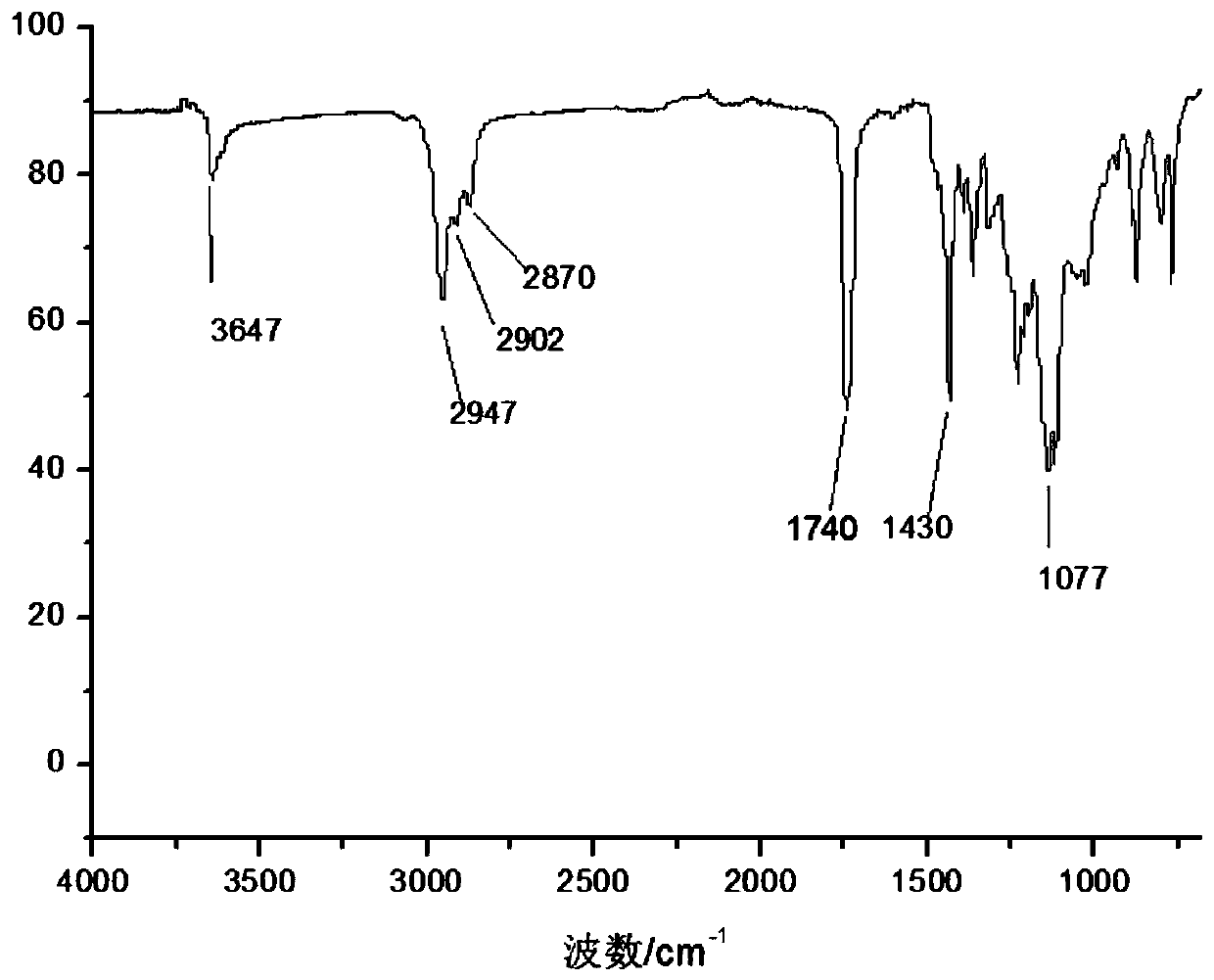
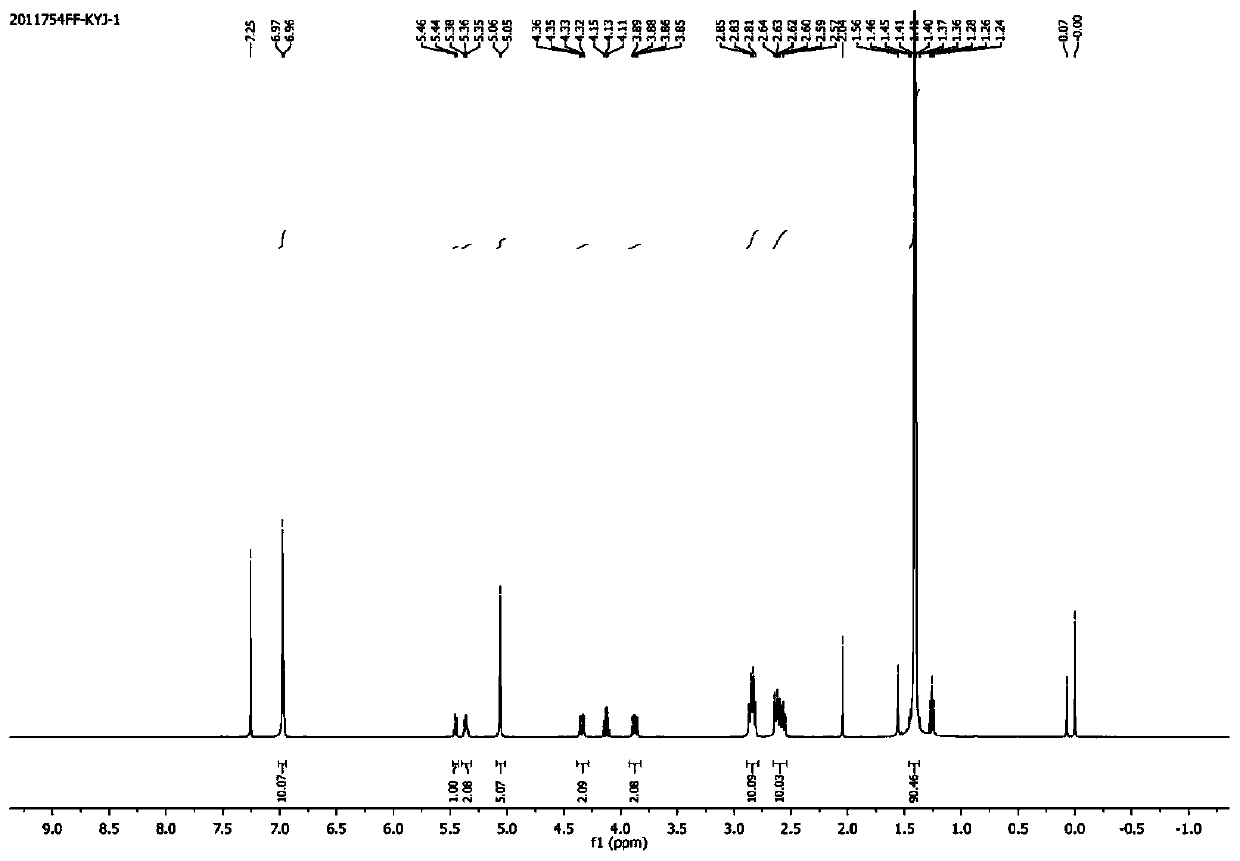
[0061] (1) Perform FTIR test and 1H-NMR test on the obtained product, the results are as follows: figure 1 with figure 2 As shown, figure 1 Is the FTIR spectrum of the product obtained in Example 1 of the present invention, figure 2 This is the 1H-NMR spectrum...
Embodiment 2
[0065] Add 84.8g (0.29mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl)methyl acrylate into a 250mL four-necked flask equipped with an electric stirrer, thermometer, condenser and methanol receiver. , Xylitol 7.6g (0.05mol), catalyst sodium methoxide 0.20g, heating and stirring, control the temperature at 190±5℃, first react at normal pressure for 6h, then react under reduced pressure for 2h, extract the methanol gas generated by the reaction, and react to No more methanol is formed. The temperature is lowered, the product is recrystallized with isopropanol, filtered, washed with isopropanol, and dried to obtain the product, which has a white solid appearance.
[0066] The product was tested according to the characterization and testing of Example 1, and the result showed that the product obtained had the structure of formula (1). The product yield was 92%, and the purity was 98.6%. The melting point of the product is 75.2~76.6℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com