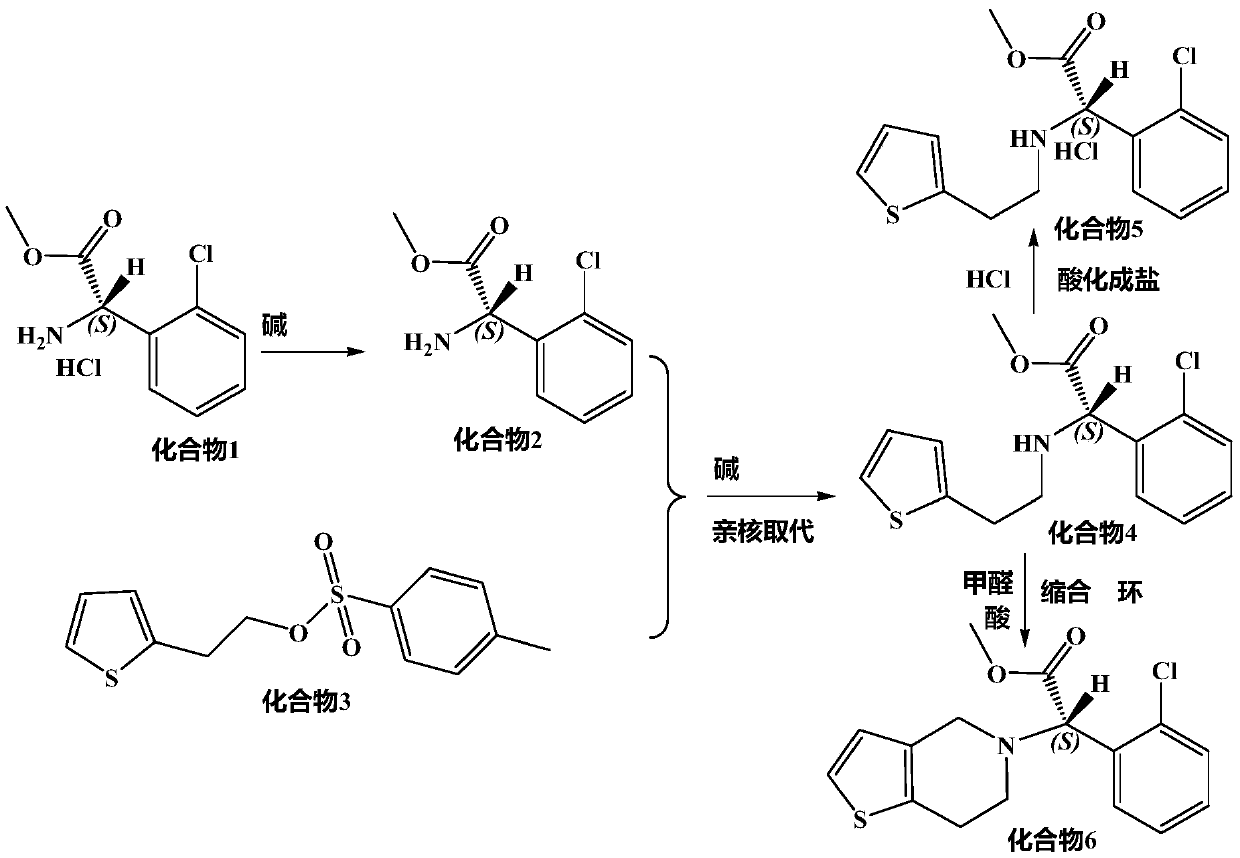
Synthesis of clopidogrel intermediate (S)-2-(2-thiopheneethylamino)-(2-chlorophenyl)-methyl acetate
A technology of thiopheneethylamine group and o-chlorophenylglycine methyl ester, which is applied in the field of medicine, can solve the problems of long nucleophilic substitution reaction time, difficult stirring, volatilization loss of reaction solvent, etc., achieves high product purity and optical purity, and overcomes the problem of solvent The effect of large volatile losses, improved yield and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Alkalinization and freeing: Weigh 86g (0.3644mol) of the raw material (S)-o-chlorophenylglycine methyl ester hydrochloride and place it in a 2000ml three-neck flask, add 300ml pure water to dissolve, add 24g sodium carbonate and 200ml pure water dropwise under stirring at room temperature The solution made is neutralized to alkaline, free (S)-o-chlorophenylglycine methyl ester, extracted 3 times with equal volume of dichloromethane, the organic phase is washed 3 times with water, the combined dichloromethane organic phase, the organic The phase was concentrated under reduced pressure to obtain (S)-o-chlorophenylglycine methyl ester concentrated solution for use.
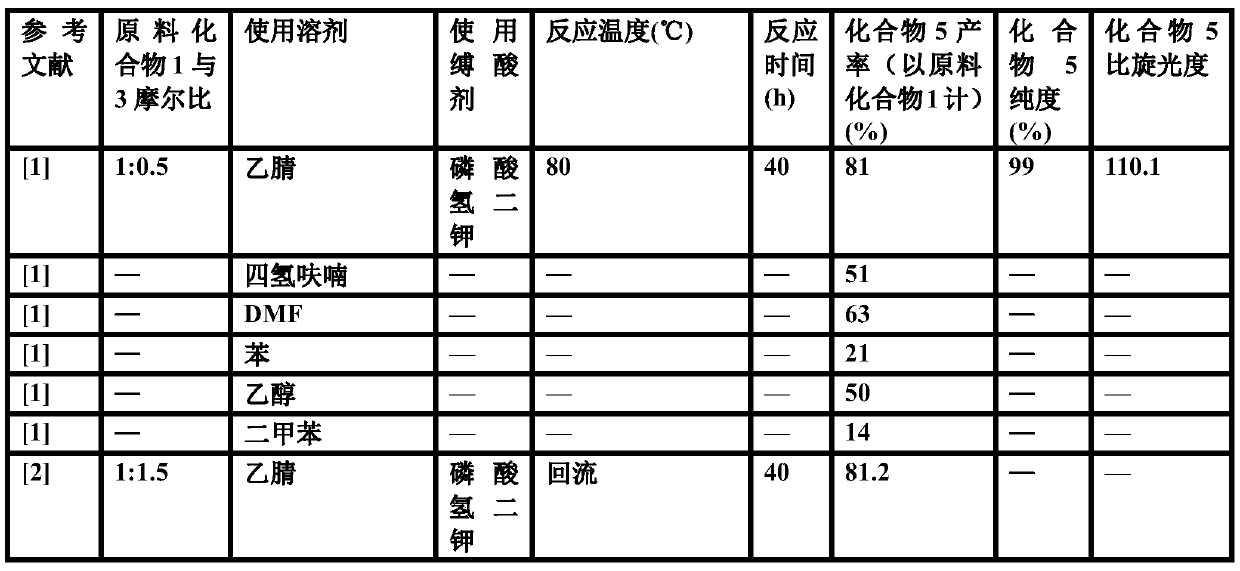
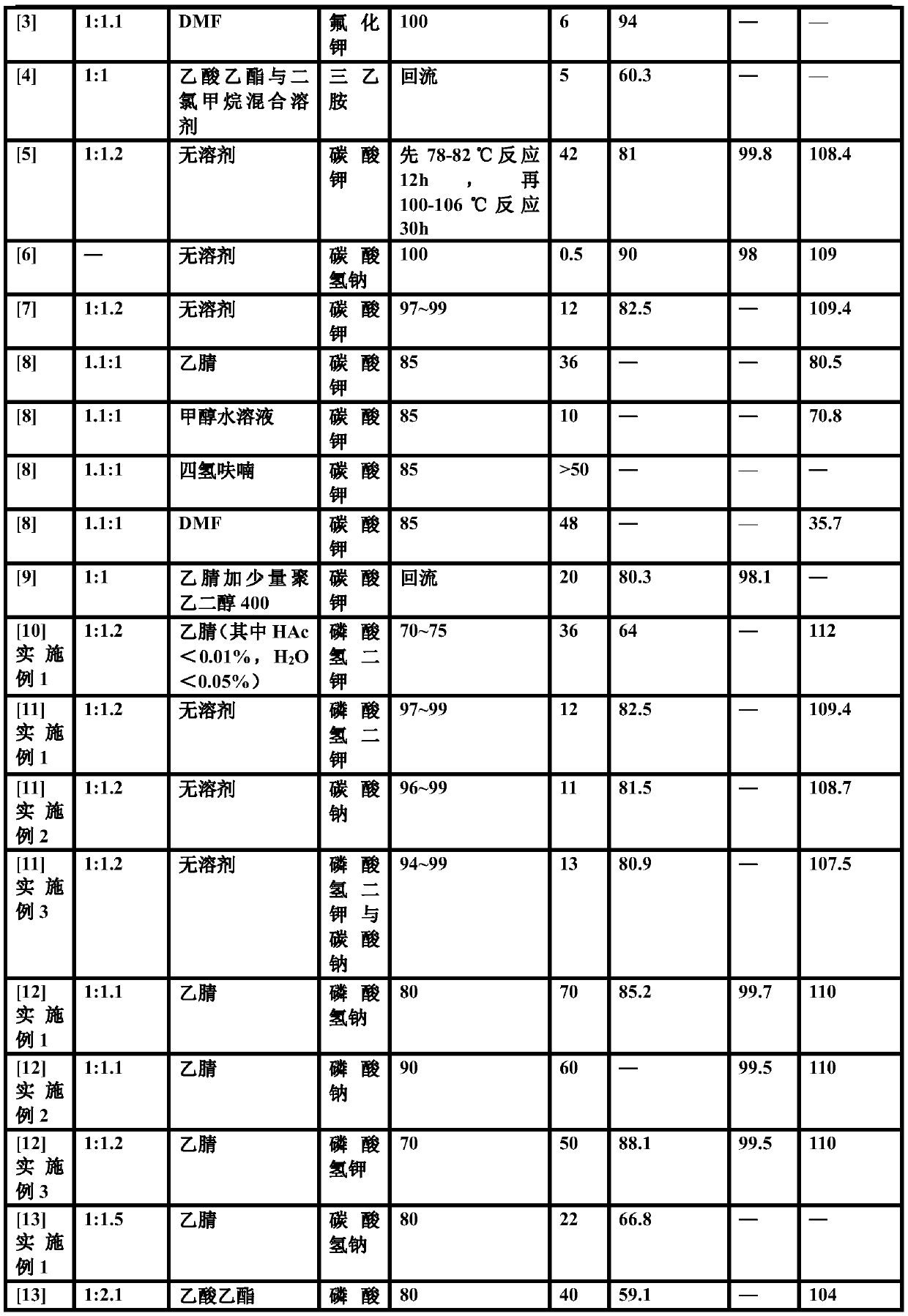
[0053] Nucleophilic substitution: put the above (S)-o-chlorophenylglycine methyl ester concentrate in a 2000ml three-neck flask, add 350g of succinonitrile, heat up to 70°C to dissolve, add the raw material thiophene-2-ethyl p-toluenesulfonate 102.8 g, stirred, and added a mixture of 160g dipotassium hydrogen ...
Embodiment 2
[0058] Alkalinization and freeing: Weigh 86g (0.3644mol) of the raw material (S)-o-chlorophenylglycine methyl ester hydrochloride and place it in a 2000ml three-neck flask, add 300ml pure water to dissolve, add 24g sodium carbonate and 200ml pure water dropwise under stirring at room temperature The solution made is neutralized to alkaline, free (S)-o-chlorophenylglycine methyl ester, extracted 3 times with equal volume of dichloromethane, the organic phase is washed 3 times with water, the combined dichloromethane organic phase, the organic The phase was concentrated under reduced pressure to obtain (S)-o-chlorophenylglycine methyl ester concentrated solution for use.
[0059] Nucleophilic substitution: put the above (S)-o-chlorophenylglycine methyl ester concentrate in a 2000ml three-necked flask, add 350g of succinonitrile, heat up to 60°C to dissolve, add the raw material thiophene-2-ethyl p-toluenesulfonate 92.5 g, stirred, and added a mixture of 160g dipotassium hydrogen...
Embodiment 3
[0062] Alkalinization and freeing: Weigh 86g (0.3644mol) of the raw material (S)-o-chlorophenylglycine methyl ester hydrochloride and place it in a 2000ml three-neck flask, add 300ml pure water to dissolve, add 24g sodium carbonate and 200ml pure water dropwise under stirring at room temperature The solution made is neutralized to alkaline, free (S)-o-chlorophenylglycine methyl ester, extracted 3 times with equal volume of dichloromethane, the organic phase is washed 3 times with water, the combined dichloromethane organic phase, the organic The phase was concentrated under reduced pressure to obtain (S)-o-chlorophenylglycine methyl ester concentrated solution for use.
[0063] Nucleophilic substitution: put the above (S)-o-chlorophenylglycine methyl ester concentrate in a 2000ml three-necked flask, add 350g of succinonitrile, heat up to 75°C to dissolve, add the raw material thiophene-2-ethyl p-toluenesulfonate 113.0 g, stirred, and added a mixture of 160g dipotassium hydroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com