Copper-based catalyst for preparing methyl glycolate and preparation and application thereof
A technology of methyl glycolate and copper-based catalysts, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as limitations and unavoidable, and achieve The effect of simple composition, increased binding capacity, energy saving and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
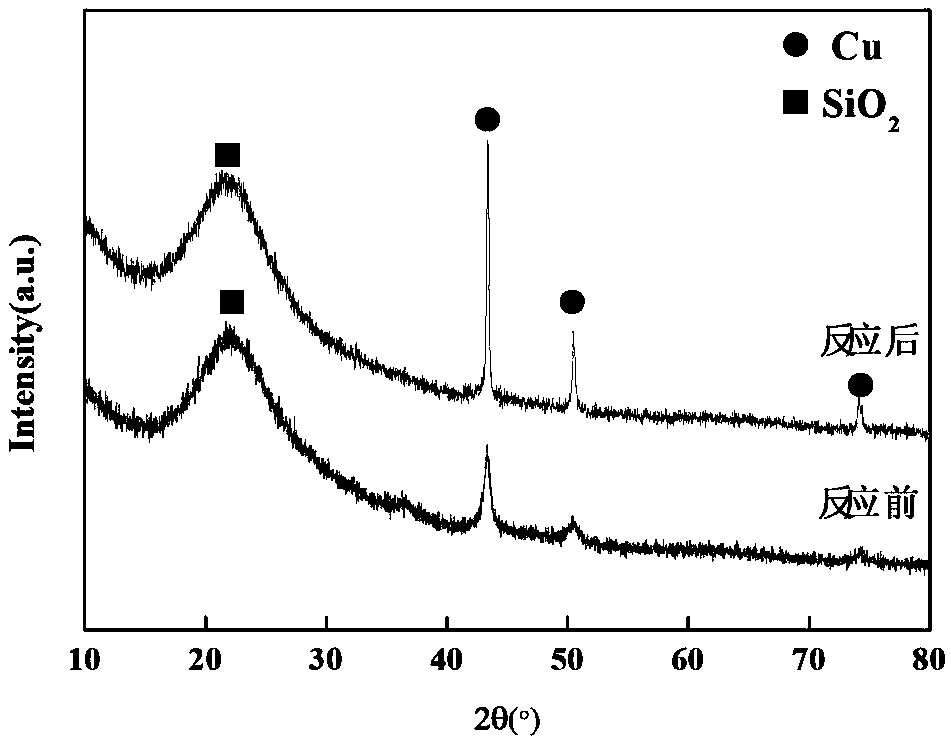
[0020] The high-purity copper (99.9%) target was pre-bombarded with Ar ions generated by a plasma generator for 30 minutes, and then the SiO 2 Carrier (specific surface area 267m 2 / g) As for the multi-angle rotating barrel, vacuumize the barrel until the pressure reaches 9.9×10 -4 Below Pa, put high-purity Ar gas into the barrel until the pressure reaches 2.0Pa, adjust the voltage of the Ar ion generator to 450V, and increase the barrel speed to 3.5rpm, so that the nano-metal copper particles produced by the Ar ion bombardment of the copper target are evenly deposited on the On the surface of the carrier, sputter for 4 hours. After the sputtering is over, the barrel is filled with 1% O by volume 2 / Ar mixture until the pressure reaches normal pressure. After testing, Cu / SiO with a mass loading of Cu of 15% was obtained 2 Catalyst, denoted as SP-15Cu / SiO 2 .
[0021] Fill the reactor with 0.5g of prepared SP-15Cu / SiO 2 Catalyst, without pre-reduction treatment, feed the ...
Embodiment 2
[0023] In the reactor, fill the SP-15Cu / SiO prepared by 0.5g embodiment 1 2 Catalyst, without pre-reduction treatment, feed the reaction raw material, H 2 / Dimethyl oxalate (DMO) molar ratio is 80, reaction temperature is 250°C, pressure is 3.0MPa, liquid hourly space velocity is 0.5h -1 , the conversion rate of dimethyl oxalate is 38.8%, the selectivity of methyl glycolate is 86.5%, the selectivity of ethylene glycol is 12.2%, the selectivity of ethanol is 0.4%, and the selectivity of C3-C4 alcohols is 0%. The yield of methyl glycolate was 33.6%.
Embodiment 3
[0025] In the reactor, fill the SP-15Cu / SiO prepared by 0.5g embodiment 1 2 Catalyst, without pre-reduction treatment, feed the reaction raw material, H 2 / Dimethyl oxalate (DMO) molar ratio is 80, reaction temperature is 260°C, pressure is 3.0MPa, liquid hourly space velocity is 0.5h -1 , the conversion rate of dimethyl oxalate is 53.8%, the selectivity of methyl glycolate is 80.4%, the selectivity of ethylene glycol is 18.6%, the selectivity of ethanol is 0.8%, and the selectivity of C3-C4 alcohols is 0.2%. The yield of methyl glycolate was 43.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com