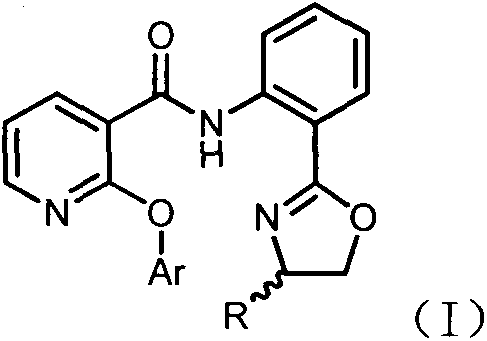
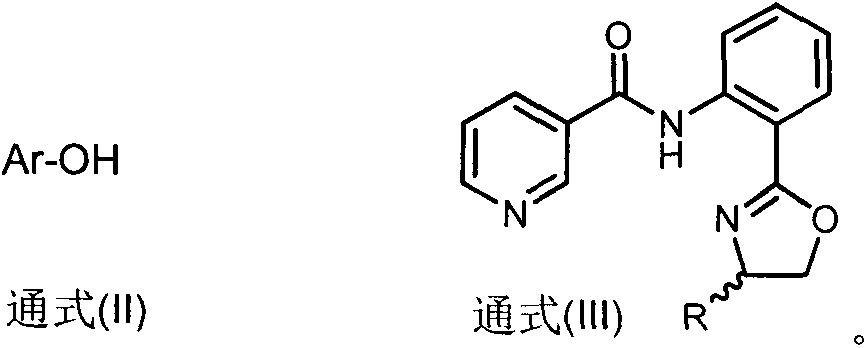
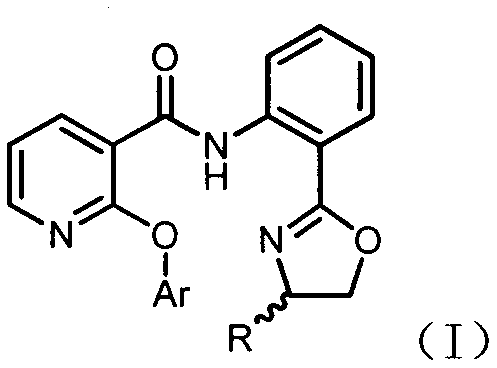
Novel 2-aryloxynicotinamide compound, and preparation method and application of same
A technology for aryloxynicotinamide and amide compounds, which is applied in the application field of synthesizing diflufenicin and 2-aryloxynicotinic acid compounds, and can solve the problem of expensive, harsh and chlorine-containing waste water waste from starting materials and other problems, to achieve the effect of easy control of the operation process, good herbicidal activity, and simple and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of Nicotinamide Compound C1
[0038]
[0039] Weigh the o-(4,5-dihydro-2-oxazolyl)aniline intermediate B1 (1mmol), niacin (1mmol), and 4-dimethylaminopyridine DMAP (0.2mmol) in a 50mL eggplant-shaped flask, Dissolve in 5 mL of dichloromethane (DCM), add condensing agent EDCI (1.2 mmol) under ice bath, stir at room temperature, thin layer chromatography (TLC) to track and monitor the reaction process, about 6h after treatment. Add 10 mL of water to quench the reaction, separate the layers and extract with dichloromethane (15 mL×3). After the dichloromethane phases are combined, they are washed with water (10 mL×2) and saturated sodium chloride solution (10 mL×2). Anhydrous sulfuric acid The sodium was dried, the solvent was evaporated under reduced pressure, and after silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 10:1), compound C1 was obtained with a yield of 74%. LC-MS(ESI+)m / z: Calcd.for[M+H: C 15 H 14 N 3 O 2 ]: 268.11, ...
Embodiment 2
[0040] Example 2: Synthesis of 2-aryloxynicotinamide compound D1
[0041] Weigh nicotinamide compound C1 (1mmol), sodium carbonate (2mmol) and copper nitrate (0.5mmol) into the reaction flask, add DMSO (5mL), and then add 3-trifluoromethylphenol ( 3mmol), react at 80°C for 8 hours, cool to room temperature, add ethyl acetate (EtOAc, 20mL) to dilute, then add (28%-30%) ammonium hydroxide (NH 3 ·H 2 O, 8mL) solution into the reaction system, continue to stir for 20min, and extract with ethyl acetate (EtOAc, 20mL×3), the organic phases were combined and washed with saturated sodium bicarbonate solution (NaHCO 3 , 10mL×3), washed with water (10mL×3), saturated sodium chloride (NaCl, 10mL×3) solution, washed the organic phase with anhydrous sodium sulfate (Na 2 SO 4 ) After drying and concentration, column chromatography (V Petroleum ether : V Ethyl acetate =12:1) to obtain a white solid D1 with a yield of 71%.
[0042]
[0043] LC-MS(ESI+)m / z: Calcd.for[M+H: C 22 H 17 F 3 N 3 O 3 ]: 4...
Embodiment 3
[0045] Example 3: Synthesis of 2-aryloxynicotinic acid compound E1
[0046]
[0047] Weigh 2-(3-trifluoromethyl)phenoxynicotinamide compound D1 (1mmol) and sodium hydroxide (40mmol) into the reaction flask, add 5mL ethanol, and react at 80℃ for 6h. After the reaction is complete, Evaporate the organic solvent, add water, extract with ethyl acetate (EtOAc) (15mL×3), combine the organic phases and add anhydrous sodium sulfate to dry, concentrate and column chromatography (eluent: V Petroleum ether : V Ethyl acetate =20:1), 125 mg of oxazolinamine B1 was recovered, and the yield was 77.6%. Add 1M hydrochloric acid (HCl) solution to the aqueous phase, adjust the pH to 4, extract with ethyl acetate (EtOAc) (15mL×3), add anhydrous sodium sulfate to dry, concentrate and column chromatography (eluent: V Petroleum ether : V Ethyl acetate = 2:1) to obtain 2-(3-trifluoromethyl)phenoxynicotinic acid E1 with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com