Annulated indigo receptor, and polymer, preparation method and applications thereof
A polymer and compound technology, applied in the field of materials, can solve problems such as the development of high-performance n-type materials and bipolar materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1, polymer P2FBAI-V
[0085] 1a) Difluoroindigo (i.e. 2F-indigo)
[0086] 30g of 5-fluoro-2-nitrobenzaldehyde (0.177mol), 1000mL of acetone and 440mL of water were successively added into the round bottom flask, and the mixture was sonicated until clear. 2mol / L sodium hydroxide (8.5g, 0.212mol) aqueous solution was slowly added dropwise under stirring. The mixture was stirred at room temperature for 48 hours. The obtained suspension was filtered, washed with water, ethanol and acetone successively, and dried to obtain a blue product (18.1 g, 68.4%).
[0087] The structural characterization data are as follows:
[0088] The product is poorly soluble in common deuterated solvents, so 1 H NMR and 13 C NMR not yet available. Mass spectrum: HREI: [M] + calcd for C 16 h 8 f 2 N 2 o 2 :298.0554,found:298.0552.
[0089] 1b) Difluorocyclized indigo (i.e. 2FBAI)
[0090] Difluoroindigo (8.0g, 26.8mmol, 1.0equiv) was dissolved in 350mL o-xylene and heated ...
Embodiment 2
[0112] Embodiment 2, polymer P2ClBAI-V
[0113] 2a) Dichloroindigo (i.e. 2Cl-indigo)
[0114] 30g of 5-chloro-2-nitrobenzaldehyde (0.162mol), 1000mL of acetone and 440mL of water were successively added into the round bottom flask, and the mixture was sonicated until clear. 2mol / L sodium hydroxide (7.8g, 0.194mol) aqueous solution was slowly added dropwise under stirring. The mixture was stirred at room temperature for 48 hours. The obtained suspension was filtered, washed with water, ethanol and acetone successively, and dried to obtain a blue product (17.7 g, 66.1%).
[0115] The structural characterization data are as follows:
[0116] The product is poorly soluble in common deuterated solvents, so 1 H NMR and 13 C NMR not yet available. Mass spectrum: HREI: [M] + calcd for C 16 h 8 Cl 2 N 2 o 2 :329.9963,found:329.9960.
[0117] 2b) Dichlorocyclized indigo (i.e. 2ClBAI)
[0118] Dichloroindigo (10.0g, 30.2mmol, 1.0equiv) was dissolved in 400mL o-xylene and he...
Embodiment 3
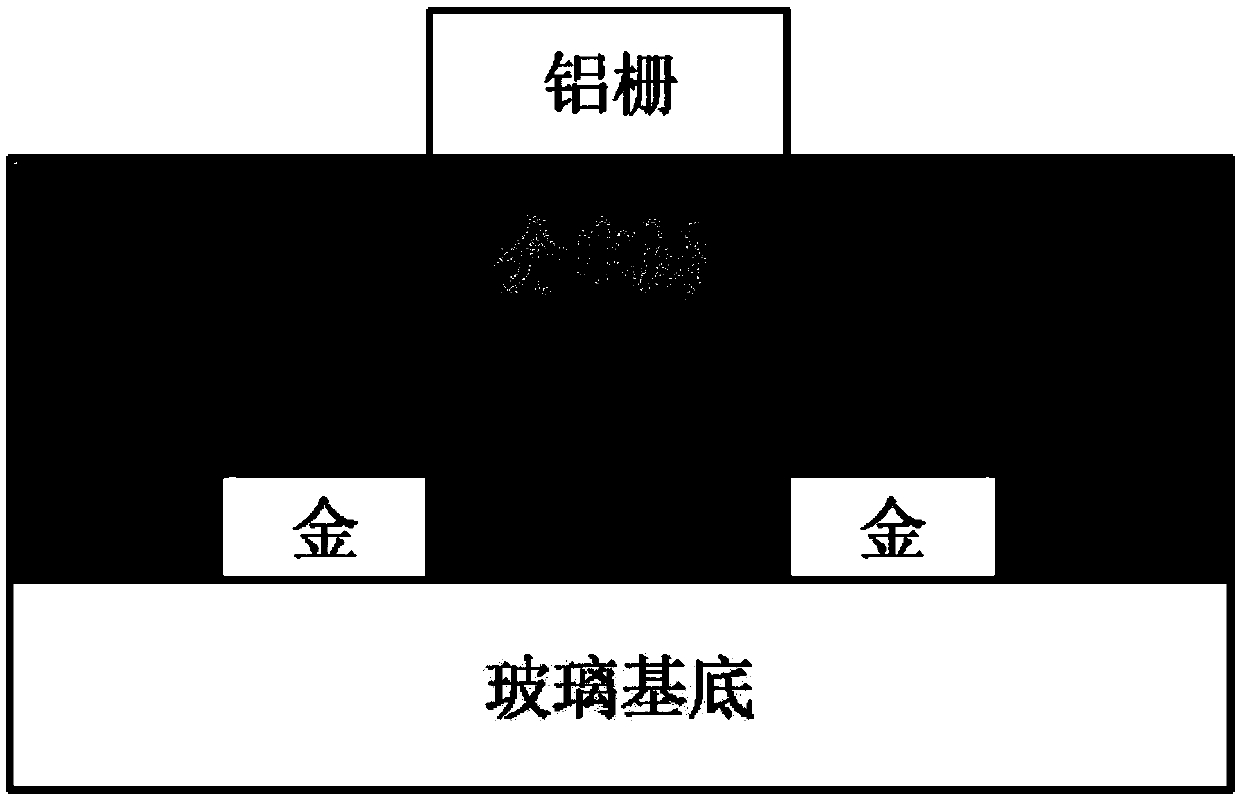
[0140] Embodiment 3, the spectral performance of polymer P2FBAI-V and P2ClBAI-V, electrochemical performance and field effect transistor performance
[0141] 1) Spectral and electrochemical properties of polymers P2FBAI-V and P2ClBAI-V
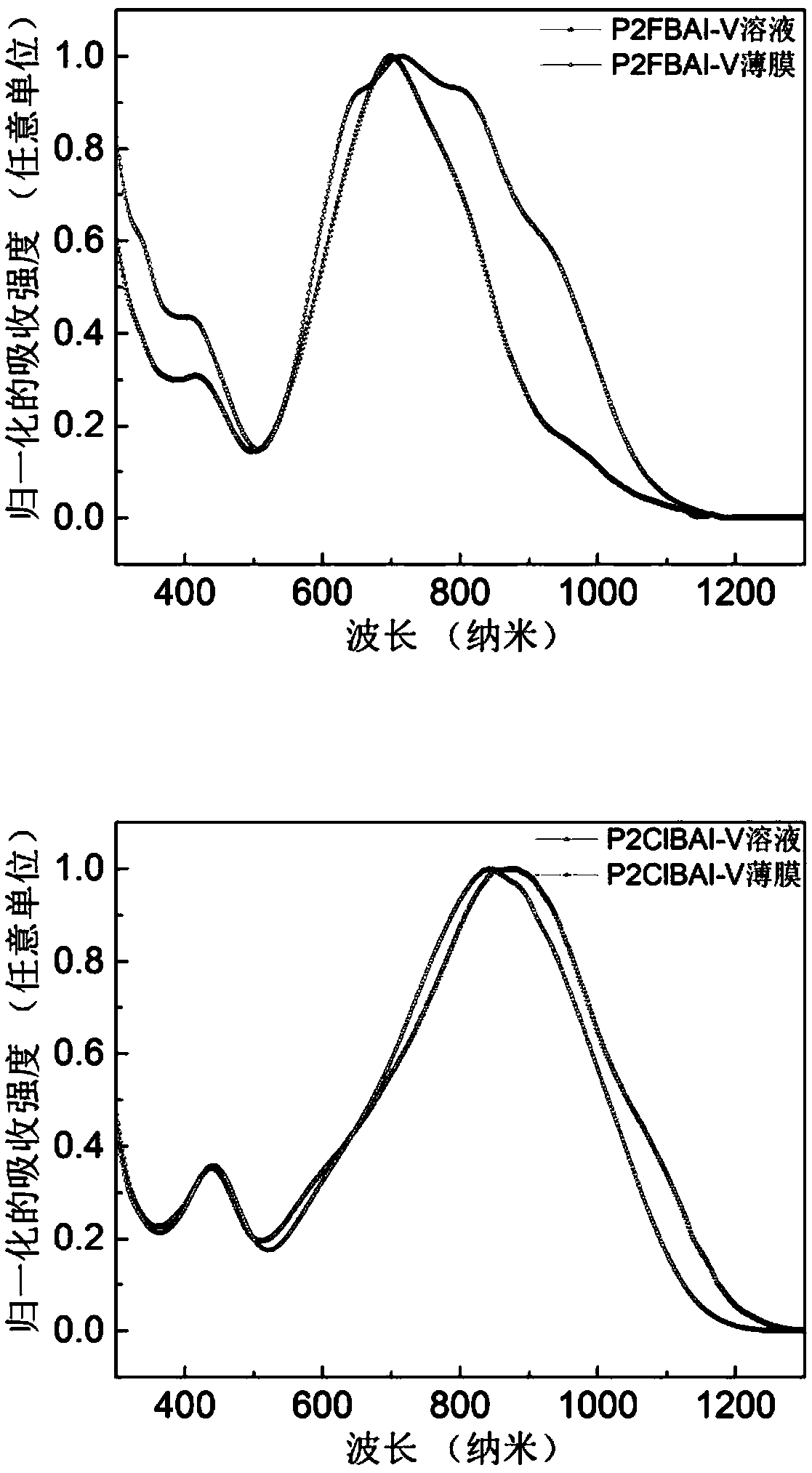
[0142] figure 1 UV-Vis absorption spectra of polymers P2FBAI-V and P2ClBAI-V in solutions and films.
[0143] Depend on figure 1 It can be seen that the optical bandgaps of polymers P2FBAI-V and P2ClBAI-V are 1.15eV and 1.09eV respectively (the optical bandgaps are based on the formula E g =1240 / λ calculation, where E g is the optical band gap, and λ is the boundary value of the UV absorption curve). Depend on figure 1 It can be seen that both polymers have relatively strong intramolecular charge transfer peaks, indicating that the intermolecular forces of the polymers are relatively strong.
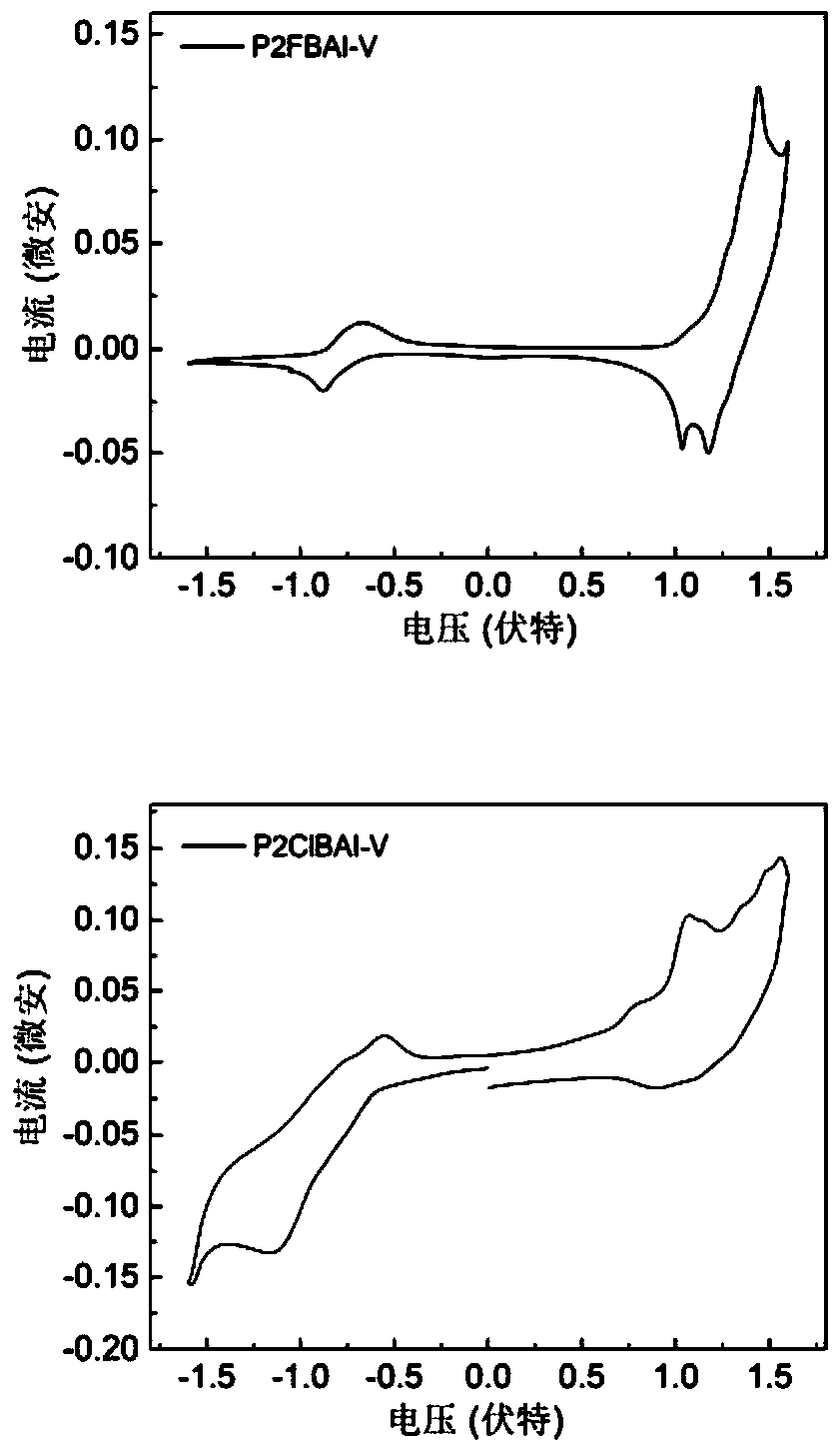
[0144] figure 2 Cyclic voltammetry curves of polymer P2FBAI-V and P2ClBAI-V films. The measurement was carried out on a CHI660c electrochemi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com