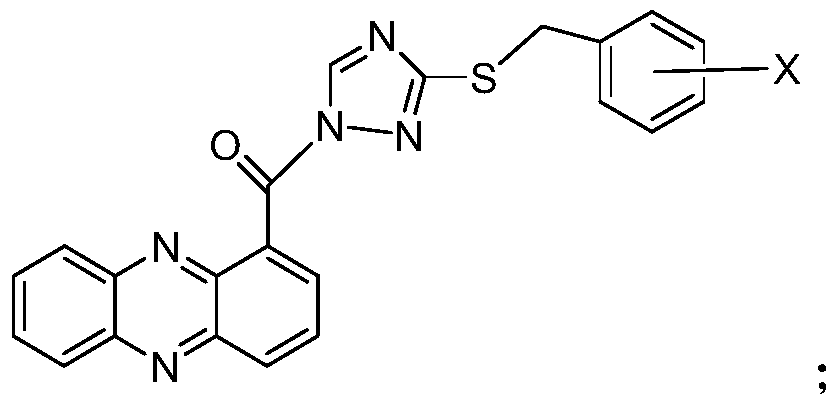
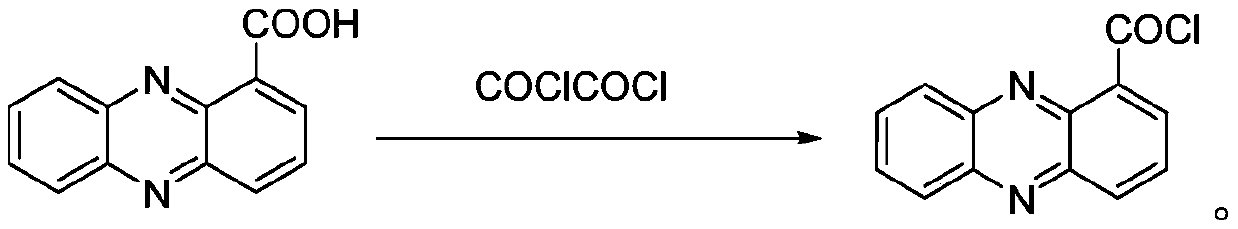Phenazino-1-carboxylic acid triazole derivative as well as preparation method and application thereof
A technology of triazole derivatives and suzinomycin, which is applied in the field of pesticide compound preparation and achieves the effect of novel chemical structure and high commercialization prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] For the second solution of the present invention, the present invention proposes a kind of synthetic method of Shenzimycin triazole derivatives, comprising the following steps: synthesizing phenazine-1-carbonyl chloride, synthesizing substituted (3-(benzylthio) )-1H-1,2,4-triazole, synthesis of substituted (3-(benzylthio)-1H-1,2,4-triazol-1-yl)(phenazin-1-yl)methanone .
[0019] The synthesis method of the above-mentioned Shenzimycin triazole derivatives is used to synthesize the Shenzimycin triazole derivatives in the first solution of the present invention.
[0020] It should be noted that in the above synthesis process, the order of synthesizing phenazine-1-carbonyl chloride and synthesizing substituted (3-(benzylthio)-1H-1,2,4-triazole can be interchanged.
[0021] Wherein, the above-mentioned synthesis of phenazine-1-carbonyl chloride is to react with sphenazine and oxalyl chloride as raw materials, and the reaction formula is as follows:
[0022]
[0023] Whe...
Embodiment 1
[0030] This embodiment provides a shenazine triazole derivative, which is the compound a02 in Table 1 and Table 2, prepared by the following steps: (1) phenazine-1-carbon Synthesis of acid chlorides
[0031] 2.24g (0.01mol) Shenzimycin, 100ml CH 2 Cl 2 Put it in a 250ml beaker, add 1.57g (0.012mol) oxalyl chloride and 3 drops of DMF dropwise with a dropper, install a condenser tube and a drying tube, turn off the condensed water, heat up to 50°C, stir and reflux for 12h, and spin the resulting solution dry, with CH 2 Cl 2 Dissolved and then spin-dried to obtain 2.2 g of a yellow-brown solid with a yield of 92%.
[0032]
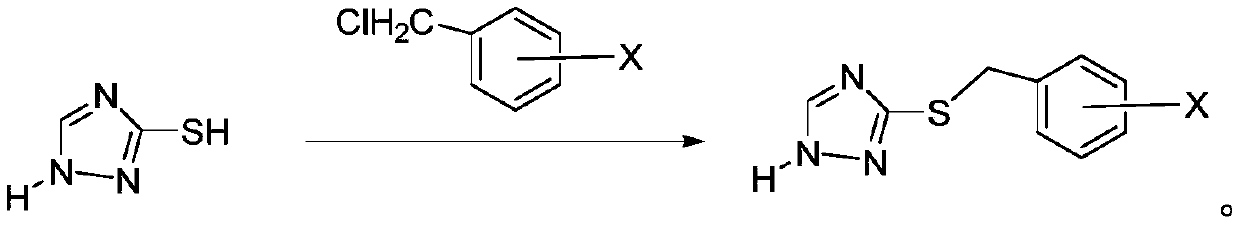
[0033] (2) Synthesis of 3-((4-methylbenzyl)thio)-1H-1,2,4-triazole.
[0034] Dissolve 2.02g (0.02mol) of 1H-1,2,4-triazole-3-thiol in a 250ml flask filled with 20ml of NaOH (4%), add 50ml of absolute ethanol, stir, and heat at 75°C for reaction , add 3.36g (0.024mol) p-methylbenzyl chloride dropwise when the color becomes lighter, keep the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com