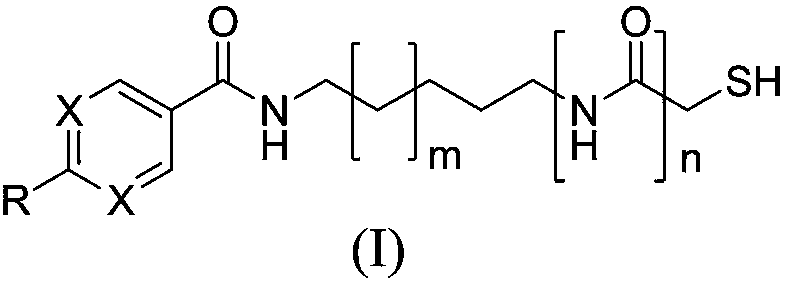
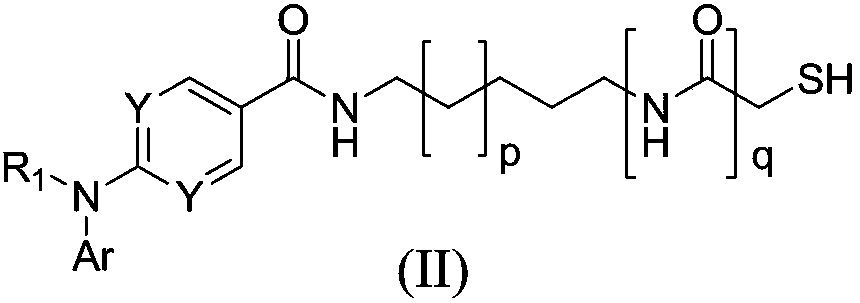
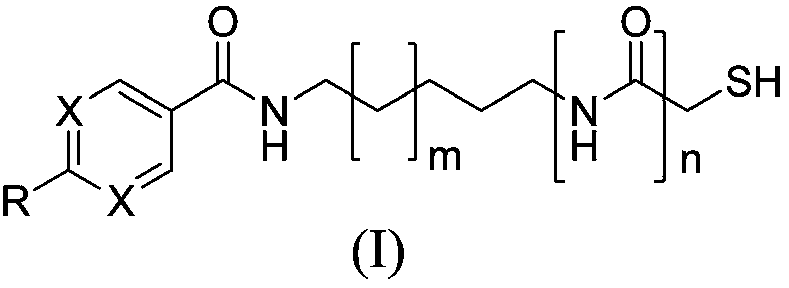
Sulfhydryl compound serving as histone deacetylase inhibitor, and application thereof
A technology of mercapto compounds and compounds, which is applied in the direction of drug combination, organic chemistry, and medical preparations containing active ingredients, etc., can solve the problems of increased Hsp90 acetylation level, strong toxic side effects, poor pharmacokinetic properties, etc., and achieve high Blood-brain barrier permeability, good selective inhibitory activity, ideal effect of pharmacokinetic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Preparation of 2-(diphenylamino)-N-(5-(2-mercaptoacetamido)pentyl)pyrimidine-5-carboxamide (I-1) and its salts
[0096]
[0097] Prepared according to Synthetic General Method 1, the synthetic route is as follows:
[0098]
[0099] Synthesis of Intermediate 2:
[0100]
[0101] Add aniline (7.58g, 81mmol), ethyl 2-chloropyrimidine-5-carboxylate (13.80g, 74mmol), potassium carbonate (20.44g, 148mmol), and DMF100ml in sequence in a 250ml three-necked flask, and store at 120°C under nitrogen protection Reaction 6h. After cooling to room temperature, the reaction solution was slowly poured into 200ml of ice-water mixture, stirred at room temperature for 30 minutes, filtered to obtain a light yellow solid, and vacuum-dried at 50°C for 12 hours to obtain 17.25g of the product with a yield of 95.88%.
[0102] Synthesis of Intermediate 3:
[0103]
[0104] Add compound 2 (17.00g, 70mmol), iodobenzene (17.11g, 84mmol), cesium carbonate (45.54g, 140mmol)...
Embodiment 2
[0127] Embodiment 2: 2-(diphenylamino)-N-(6-mercaptohexyl) pyrimidine-5-carboxamide (I-2) and its salt preparation
[0128]
[0129] Prepared according to Synthetic General Method 2, the specific synthetic route is as follows:
[0130]
[0131] Synthesis of Intermediate 5
[0132]
[0133] Add compound 4 (1.50g, 5mmol), 6-bromo-1-aminohexane hydrobromide (2.69g, 10mmol), EDC (1.97g, 10mmol), diisopropylethyl Amine (2.02g, 16mmol), THF 30ml. Stir at room temperature for 12 h, add 50 ml of water, extract 3 times with 50 ml of ethyl acetate, combine the organic layers, wash with 100 ml of saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure to dryness to obtain a pale yellow oil, column chromatography (petroleum ether : Ethyl acetate = 2: 1) Purification gave 1.65 g of light yellow oily substance, yield 71.12%.
[0134] Synthesis of Intermediate 6
[0135]
[0136] Compound 5 (1.50 g, 3 mmol), potassium thioacetate (1.51 g, 13 mmo...
Embodiment 3
[0142] Example 3 Preparation of 4-(diphenylamino)-N-(5-(2-mercaptoacetamido)pentyl)benzamide (I-3) and its salts
[0143] Preparation I-3 was carried out according to General Method 1.
[0144] 1 H NMR (400MHz, DMSO-d6) δppm: 1.32 (2H, q, J = 8.1Hz), 1.66 (4H, dp, J = 20.0, 7.8Hz), 1.97 (1H, s), 3.32 (4H, dt, J=15.2,7.7Hz), 3.45(2H,s),6.06(1H,s),6.29(1H,s),6.96 (2H,tt,J=7.4,2.0Hz),7.04–7.11(4H,m ),7.20–7.28(4H,m),7.32–7.37(2H,m),7.63–7.69(2H,m); ESI-MS(+)m / z=448.3[M+H] +
[0145] The preparation of compound 1-3 malate:
[0146] Compound I-1 (0.1 mmol) and malic acid (0.8 mmol) were added to ethanol (10 mL), dissolved under reflux, and a white solid was precipitated by cooling, and filtered to obtain 0.12 g of white I-3 malate solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com