Recombinant cl7-cvn protein and its preparation method and application
A CL7-CVN and protein technology, applied in the field of recombinant CL7-CVN protein and its preparation, can solve the problems of difficulty in renaturation of inclusion body protein, lack of fusion expression amino acids, and low expression yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The construction of embodiment 1 recombinant CL7-CVN protein prokaryotic expression vector
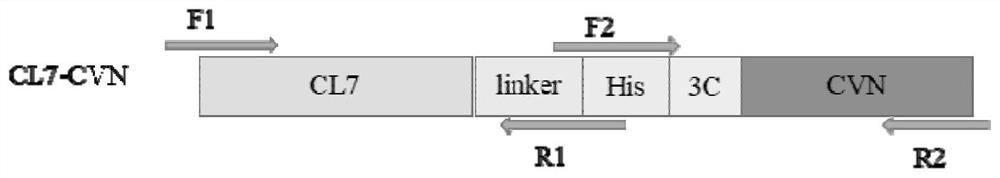
[0038] 1. The first PCR amplification: the mutant CL7 gene was obtained by site-directed mutation (the nucleotide sequence is shown in SEQ ID NO: 3, and the amino acid sequence of the mutant protein is shown in SEQ ID NO: 4);
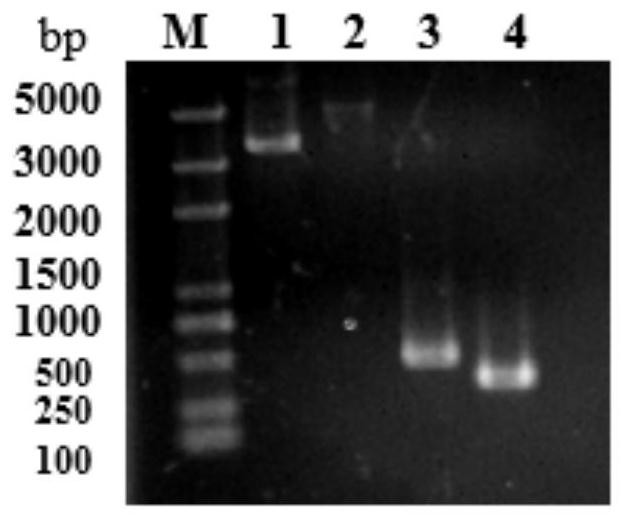
[0039] Using the pET23a-CL7 plasmid as a template, F1 shown in SEQ ID NO: 6 and R1 shown in SEQ ID NO: 7 were used as primers to simultaneously introduce linker, His and terminal homologous sequences for PCR amplification to obtain CL7- linker-His linear fragment, see image 3 Middle lane 3; according to the CVN gene sequence announced by the gene bank, carry out codon optimization design and synthesis of the CVN gene, and use the constructed pET28a-CVN vector as a template, with F2 as shown in SEQ ID NO:8 and as SEQ ID NO: R2 shown in 9 is a primer, and 3C and terminal homologous sequences are introduced at the same time, and PCR amplification is perfor...
Embodiment 2
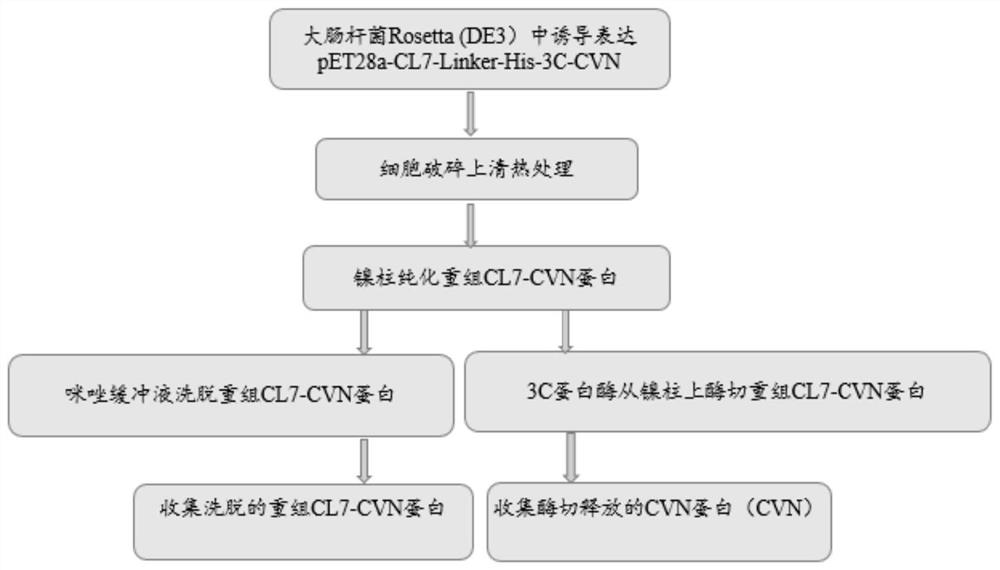
[0049] Example 2 Induced expression of recombinant CL7-CVN protein and optimization of expression conditions
[0050] The recombinant plasmid pET28a-CL7-linker-His-3C-CVN constructed in Example 1, the empty plasmid pET28a, and the control plasmid pET28a-CVN were respectively transformed into Escherichia coli Rosetta (DE3) to obtain expression strains. The expression strain was inoculated in 6ml of liquid LB medium containing kanamycin, and cultured on a shaker at 37°C at 220rpm. When the OD600 value was 0.6, the inducer IPTG was added, and the induction culture was continued on a shaker at 37°C at 220rpm. The recombinant protein expression was detected by SDS-PAGE electrophoresis method, the results are shown in Figure 6 , the induced pET28a-CL7-linker-His-3C-CVN (amino acid sequence shown in SEQ ID NO: 1) has an obvious target band at about 28kDa, which is consistent with the expected size, and the expressed protein is soluble; The CL7-labeled control pET28a-CVN showed the ...
Embodiment 3
[0055] The stability study of embodiment 3 recombinant CL7-CVN protein
[0056] Inoculate pET28a-CL7-linker-His-3C-CVN expression strains into 200ml liquid LB medium containing kanamycin, culture at 37°C until OD600 value is 0.6, add final concentration 1mM / LIPTG, and incubate at 18°C Collect the bacteria after 20 hours of induction on a shaking table; resuspend the bacteria with 20ml of lysate, break the bacteria by ultrasonic, and collect the supernatant by centrifugation; take 500ul of the supernatant in EP tubes, and place them in water bath for 15min and 30min at 90°C and 100°C respectively , 45min, 60min, 75min, 90min, 105min, 120min, the supernatant was collected by centrifugation, protein samples were prepared, and the stability of the recombinant protein was detected by SDS-PAGE electrophoresis. see results Figure 10(A, B), after 90℃, 100℃ high temperature treatment for 2 hours, most of the miscellaneous proteins were denatured, but the recombinant CL7-CVN protein w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com