Method for recovering bromine from bromine-containing wastewater in production of brominated aniline
A technology of bromoaniline and wastewater, applied in the field of bromine recovery, to achieve the effects of low equipment corrosion resistance and automation requirements, low wastewater requirements, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
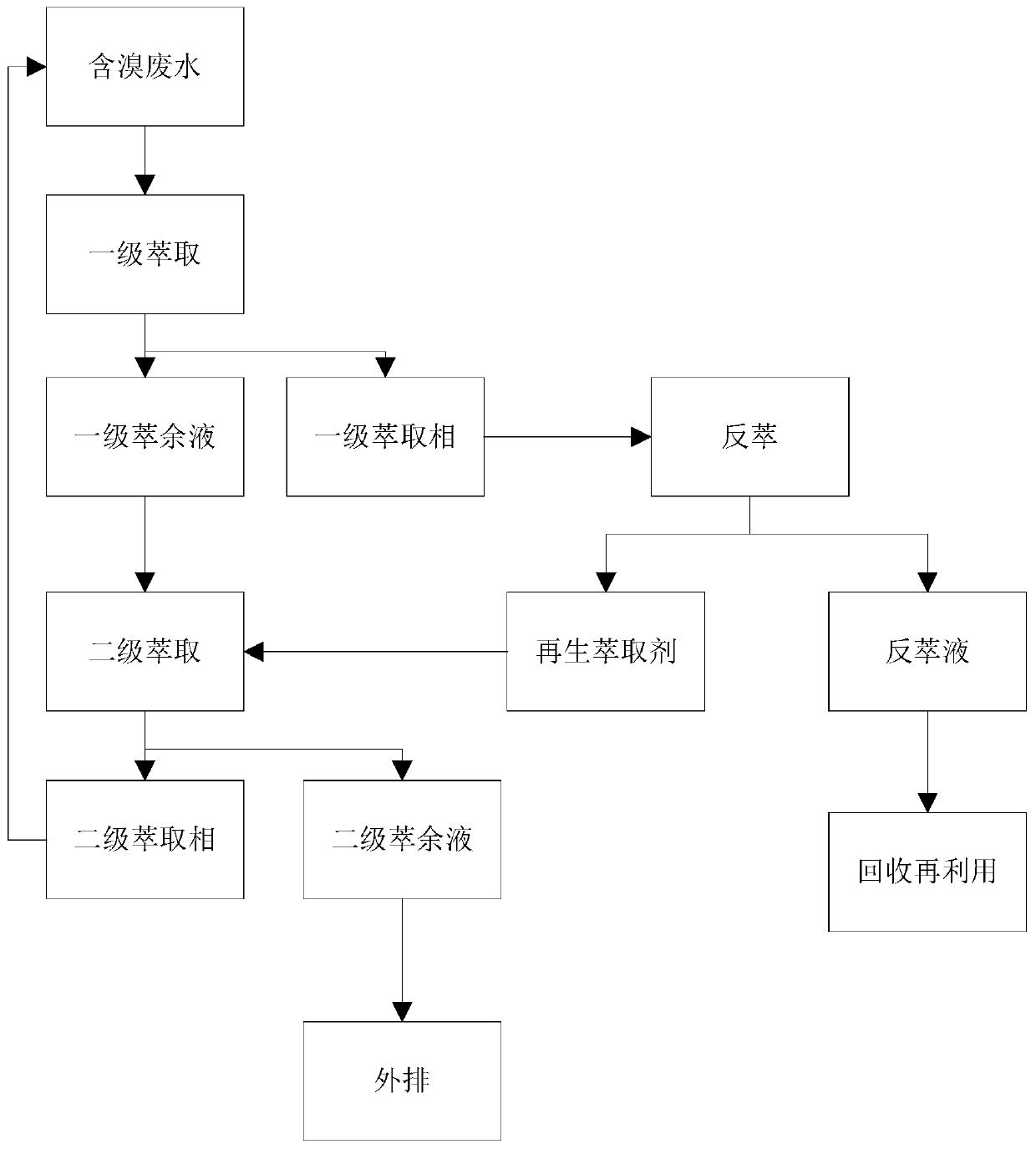
[0075] A large dye company in Zhejiang produces 2,4-dinitro-6-bromoaniline. The bromine ion content in the bromine-containing wastewater is 9640mg / L, the COD is 5040mg / L, and the acidity is 4.75%. In the present invention, acidity can be expressed as the mass fraction of sulfuric acid present in the hydrolyzate.
[0076] The method for reclaiming bromine from the bromine-containing wastewater producing 2,4-dinitro-6-bromoaniline of the present embodiment may further comprise the steps:
[0077] Step 1. Mix trioctylamine, sulfonated kerosene, and n-octanol in a volume ratio of 3:6:1 as an extractant.
[0078] Step 2. Mix waste water and extractant at a volume ratio of 2.5:1, and fully stir at 50°C for 30 minutes.
[0079] Step 3, the solution is placed in a separatory funnel, allowed to stand for stratification, and the primary extract phase (oil phase) and primary raffinate (water phase) are separated. Among them, the bromide ion concentration in the primary raffinate is 25...
Embodiment 2
[0086] A dye company in Zhejiang produces 2,6-dibromo-4-nitroaniline. The bromine ion content in the bromine-containing wastewater is 11640mg / L, the COD is 8580mg / L, and the acidity is 4.33%.
[0087] The present embodiment reclaims the method for bromine from the bromine-containing waste water of 2,6-dibromo-4-nitroaniline comprising the following steps:
[0088] Step 1. Mix trioctylamine, sulfonated kerosene and n-octanol in a volume ratio of 3:6:1 as an extractant.
[0089] Step 2. Mix the waste water and the extractant at a volume ratio of 2:1, and fully stir at 50°C for 30 minutes.
[0090] Step 3, the solution is placed in a separatory funnel, allowed to stand for stratification, and the primary extract phase (oil phase) and primary raffinate (water phase) are separated. The bromide ion concentration in the primary extract is 4322mg / L, and the COD is 5690mg / L.
[0091] Step 4. The primary raffinate (aqueous phase) is then mixed with the extractant at a volume ratio of...
Embodiment 3
[0097] A dye company in Zhejiang produces p-bromoaniline. The bromine ion content in the bromine-containing wastewater is 7450mg / L, the COD is 4269mg / L, and the acidity is 2.68%.
[0098] Step 1. Mix trioctylamine, sulfonated kerosene, and n-octanol in a volume ratio of 3:6:1 as an extractant.
[0099] Step 2. Mix waste water and extractant at a volume ratio of 2.5:1, and fully stir at 50°C for 30 minutes.
[0100] Step 3, the solution is placed in a separatory funnel, allowed to stand for stratification, and the primary extract phase (oil phase) and primary raffinate (water phase) are separated. The bromide ion concentration in the primary extract is 1760mg / L, and the COD is 1346mg / L.
[0101] Step 4. The primary raffinate (aqueous phase) is then mixed with the extractant at a volume ratio of 2.5:1 for secondary extraction, and the extraction conditions are the same as in step 2.
[0102] Step 5, obtain secondary extraction phase (oil phase) and secondary raffinate (water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com