Oxygen reduction catalyst and preparation method thereof
A catalyst, cobalt oxide technology, applied in the field of electrochemistry, can solve the problems of low precious metal platinum reserves, limited metal-air batteries and fuel cells, high price, and achieve good half-wave potential effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of copper-cobalt-nickel-based nitrogen-doped carbon nanotubes
[0030] (1) Synthesis of copper-cobalt oxide
[0031] Put nickel foam with a length of 3 cm and a width of 1 cm in 40 mL of 10% hydrochloric acid, and ultrasonically clean it for 15 minutes in an ultrasonic cleaner.
[0032] Weigh 0.2428 g of copper nitrate, 0.2939 g of cobalt nitrate, and 0.5351 g of urea into a beaker, add 60 mL of deionized water, and stir for 1 h at room temperature. When the stirring is completed, the solution is a uniform pink solution. The stirred solution and the cleaned foamed nickel were placed in a Teflon-lined autoclave, and hydrothermally reacted in an oven at 120 °C for 12 h to obtain a copper-cobalt oxide nanosheet composite. Rinse 3-5 times respectively, and then dry in an oven at 60°C.
[0033] (2) Preparation of copper-cobalt-nickel-based nitrogen-doped carbon nanotubes
[0034] The copper-cobalt oxide obtained in step (1) was calcined at 400 °C for...
Embodiment 2
[0035] Example 2 Composition and structural characteristics of copper-cobalt-nickel-based nitrogen-doped carbon nanotubes
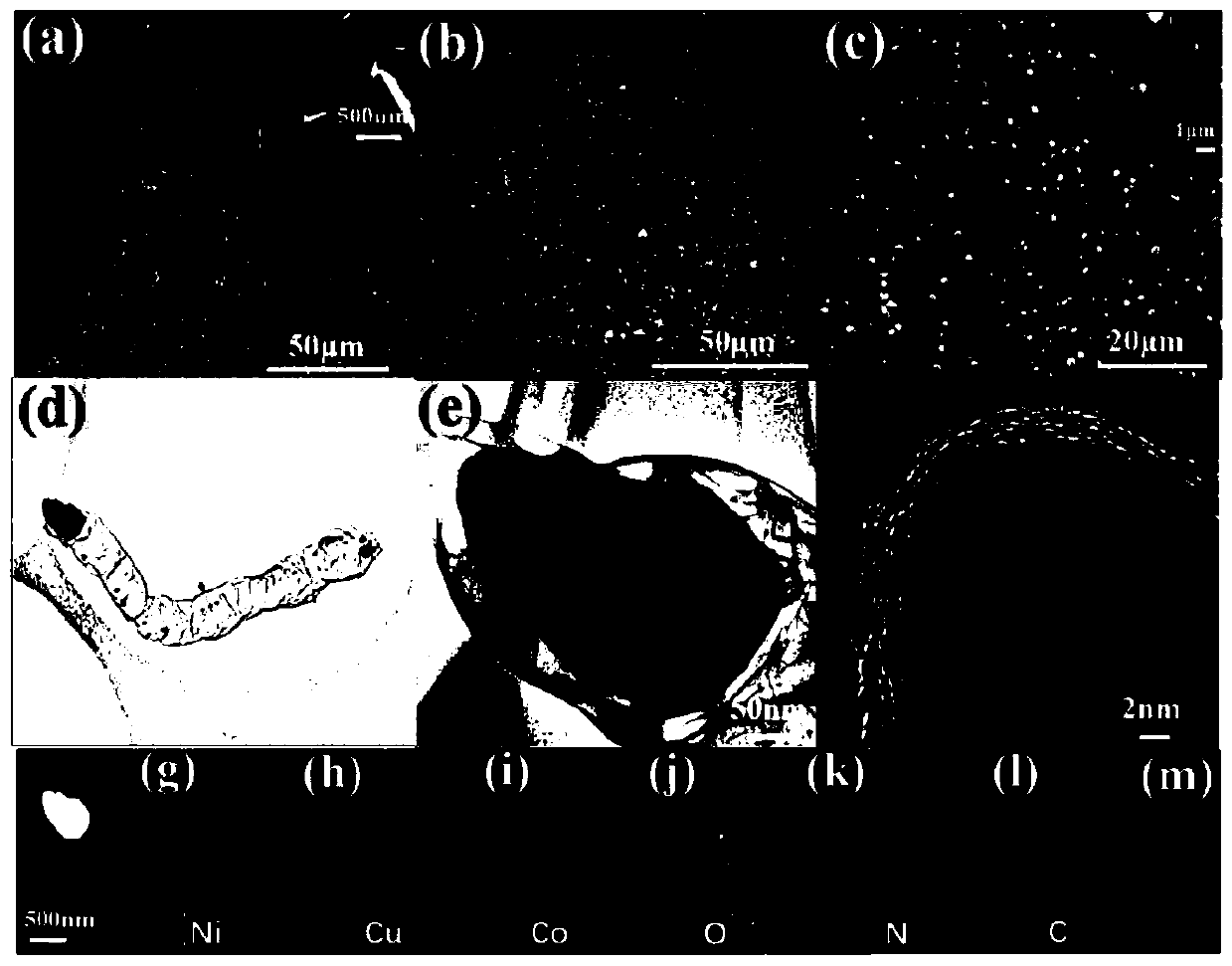
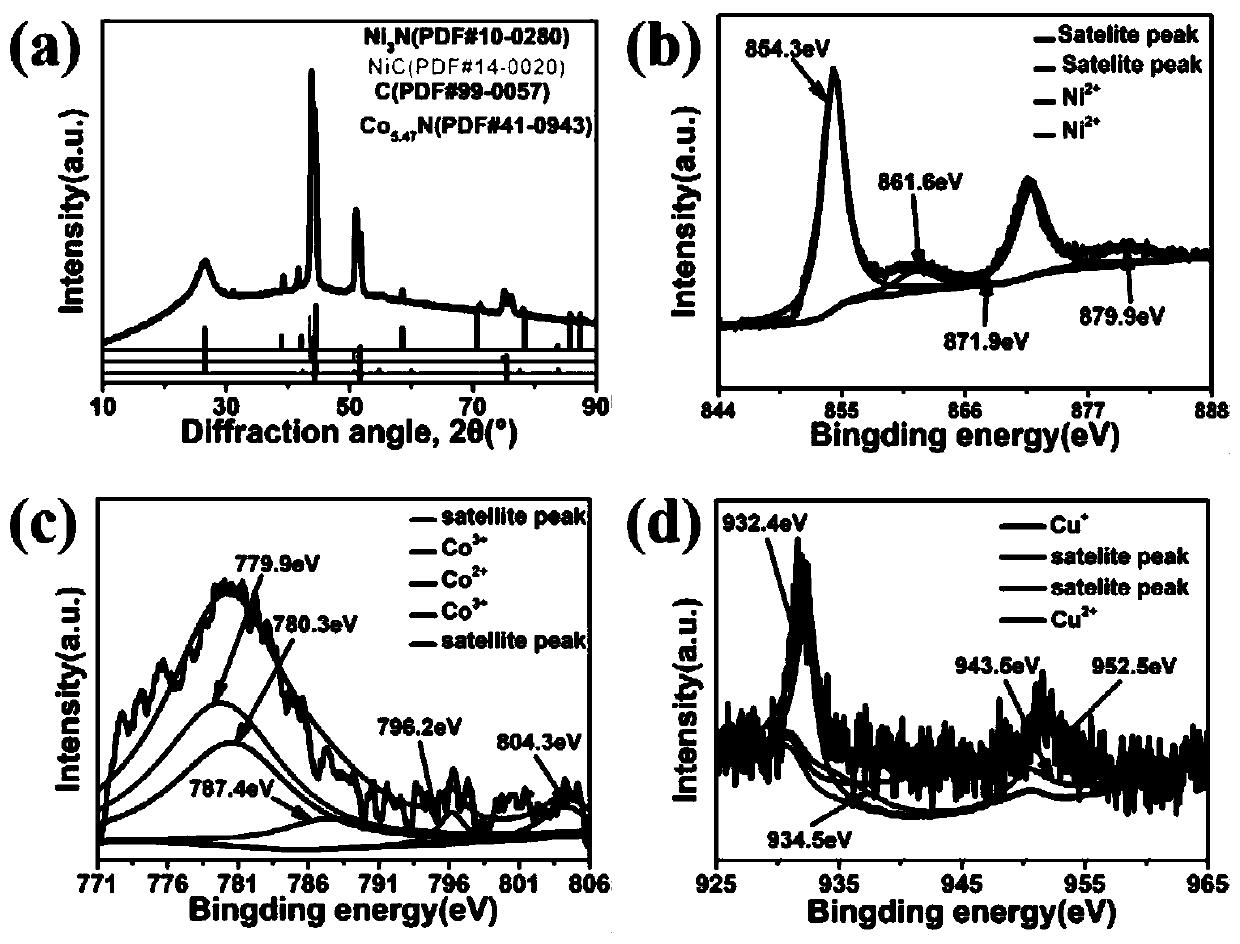
[0036] Scanning electron microscope images such as figure 1 shown in a-c. After hydrothermal treatment, the nickel foam skeleton has a uniform copper-cobalt oxide nanosheet composite with a length of about 1 μm, which makes the surface of the nickel foam nanoscale. After calcination, nanotubes embedded with transition metal nanoparticles are obtained, and the nanoparticles cover the top of each nanotube, as figure 1shown in d-f; figure 1 The lattice distance in f is about 0.235 nm, corresponding to the C(100) plane. The average diameter of these carbon nanotubes is 800 nm. According to the element map, such as figure 1 As shown in g-m, it can be seen that copper, cobalt, and nickel nanoparticles are not only distributed on the tip or inside, but also uniformly distributed on the surface of carbon nanotubes. At 520 °C and 540 °C, high-temperature der...
Embodiment 3
[0038] Example 3 Electrochemical properties of copper-cobalt-nickel-based nitrogen-doped carbon nanotubes
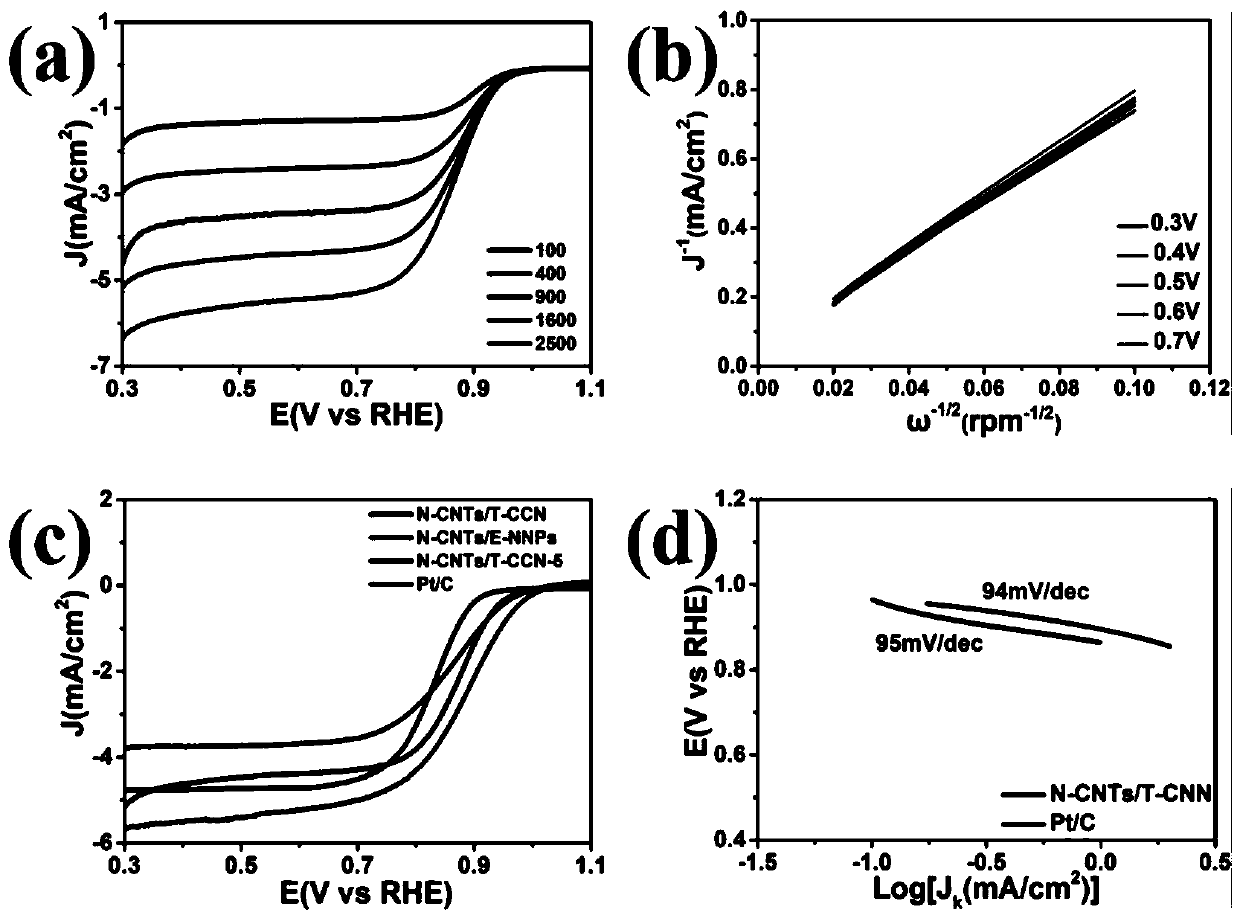
[0039] The electrochemical performance of copper-cobalt-nickel based nitrogen-doped carbon nanotubes was studied. The linear sweep voltammetry curves at different scan rates are as follows: image 3 as shown in a. Depend on image 3 a It can be seen that the catalyst has a high oxygen reduction activity, with an initial voltage of 0.96eV and a half-slope voltage of 0.87eV at a rotational speed of 1600 rpm. Notably, its catalytic activity for the oxygen reduction reaction is higher than that of some previously reported transition metal and nitrogen-doped carbon materials. Depend on image 3 b It can be seen that the Koutecky−Levich (K-L) curve at the corresponding potential shows an approximately parallel linear relationship, and the value of the electron transfer number (n) is 3.77-4.00, indicating that there is a complete four-electron transfer path. Depend on im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com