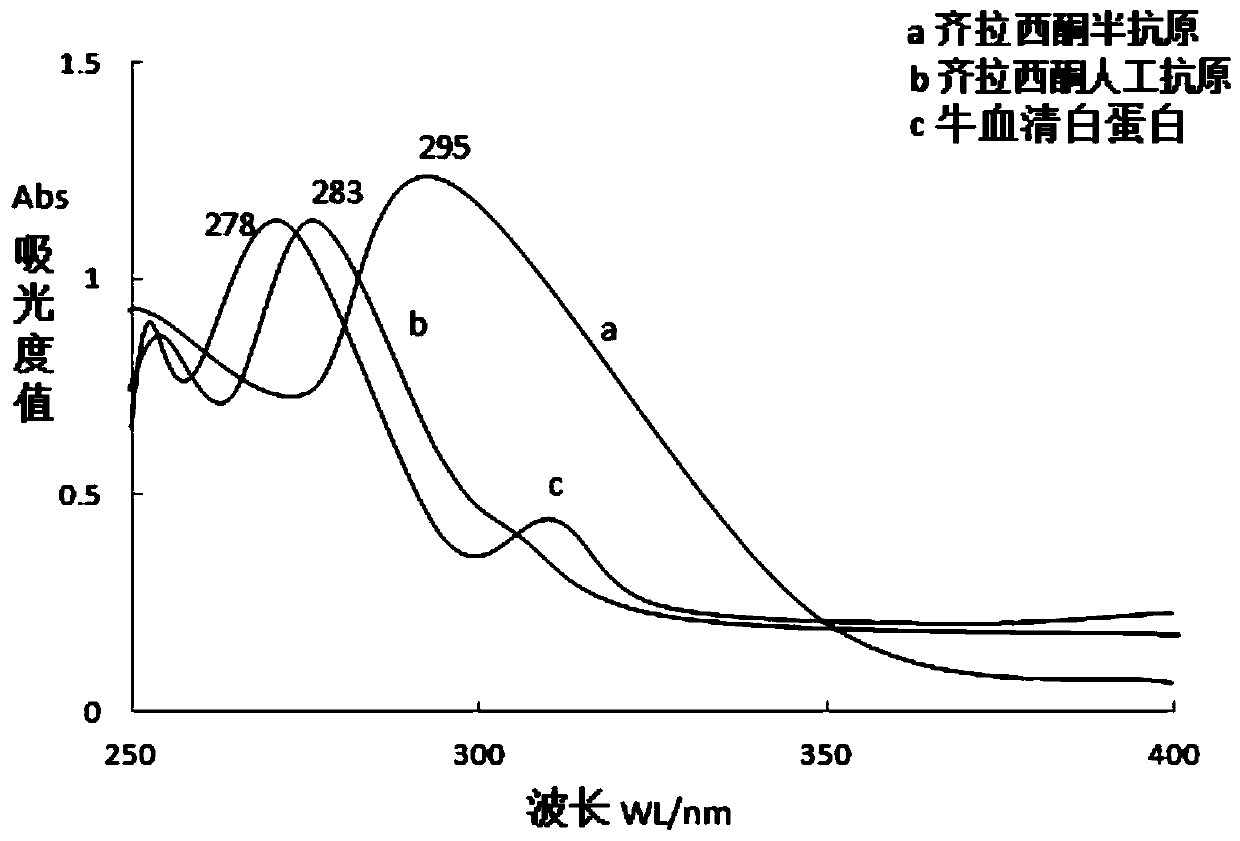
Ziprasidone artificial antigen and preparation method thereof
A technology of ziprasidone and artificial antigen, which is applied in the field of ziprasidone artificial antigen and its preparation, can solve the problems of being expensive and requiring professional and technical personnel to operate, and achieve saving time and labor costs, low price, and reducing non-specific Effects of opposite-sex unions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of ziprasidone hapten
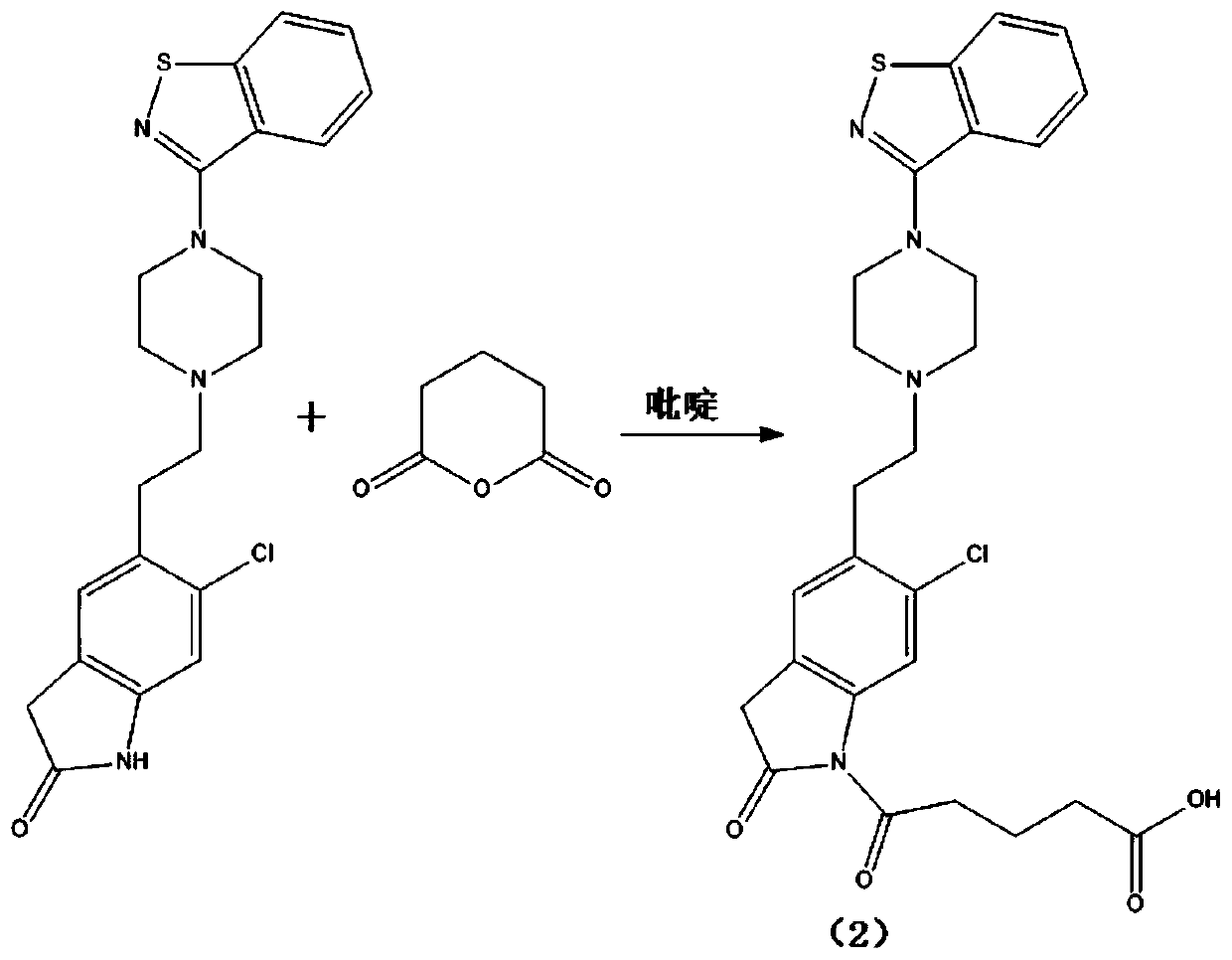
[0039] Weigh 100mg (0.24mmol) ziprasidone, add it to a 50ml single-necked round-bottomed flask, then add 5ml pyridine, 50mg (0.44mmol) glutaric anhydride, add a stirrer, stir and react at room temperature for 20 hours; , the solvent was evaporated to dryness under reduced pressure, purified by thin layer chromatography, TLC: the chromatographic solution was ethyl acetate, and the product ratio shift value Rf=0.2~0.3; the hapten (2) 75mg was obtained, and the specific synthetic route was as follows figure 1 shown.
[0040] (2) Preparation of ziprasidone artificial antigen
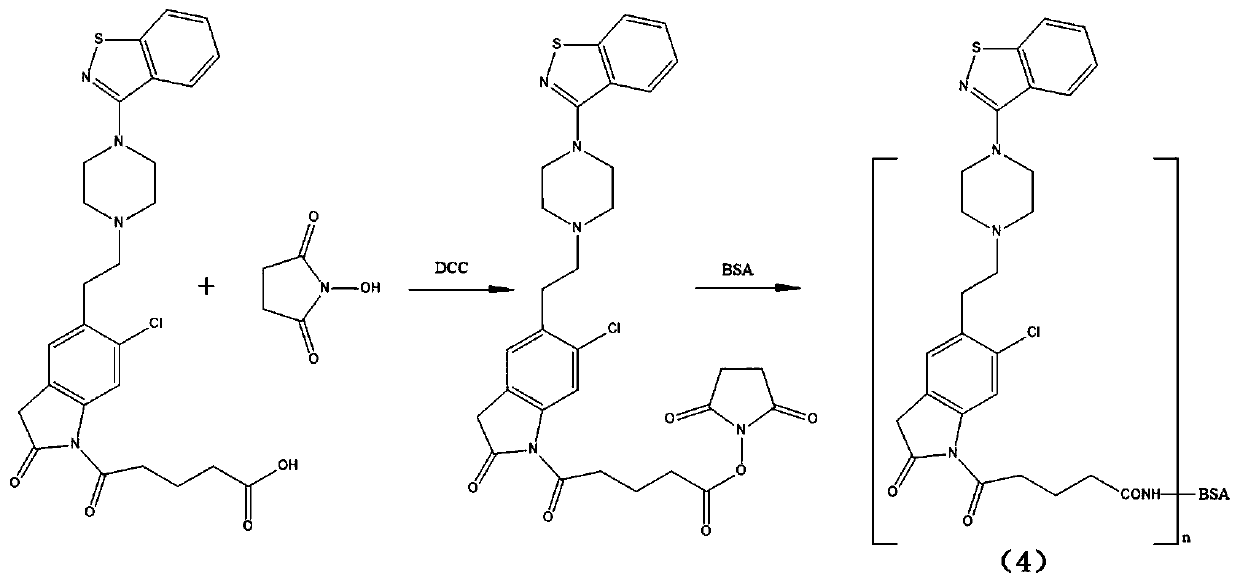
[0041] a. Weigh 50mg (0.09mmol) ziprasidone hapten in a 50ml round bottom flask, add 3ml N,N-dimethylformamide (DMF), then add 64mg (0.57mmol) N-hydroxysuccinyl Amine (NHS) and 114mg (0.55mmol) N,N-dicyclohexylcarbodiimide (DCC), stirred at room temperature overnight, centrifuged after the reaction, and the supernatant was taken as solution A.
[0042] b. Wei...
Embodiment 2
[0055] (1) Preparation of ziprasidone hapten
[0056] Weigh 100mg (0.24mmol) ziprasidone, add it to a 50ml single-necked round bottom flask, then add 5ml pyridine, 0.24mmol glutaric anhydride, add a stirrer, stir and react at room temperature for 22 hours; after the reaction, depressurize Evaporate the solvent to dryness, purify by thin-layer chromatography, TLC: the chromatographic solution is ethyl acetate, the product ratio shift value Rf=0.2~0.3; obtain hapten (2) 85 mg, the specific synthetic route is as follows figure 1 shown.
[0057] (2) Preparation of ziprasidone artificial antigen
[0058] a. Weigh 0.15mmol of hapten in a 50ml round bottom flask, add 4ml of N,N-dimethylformamide (DMF), then add 0.15mmol of N-hydroxysuccinimide (NHS) and 0.15mmol of N,N-di Cyclohexylcarbodiimide (DCC), stirred overnight at room temperature, centrifuged after the reaction, and the supernatant was taken as liquid A.
[0059] b. Weigh 14.5 g of disodium hydrogen phosphate dodecahydrat...
Embodiment 3
[0065] (1) Preparation of ziprasidone hapten
[0066] Weigh 0.20mmol ziprasidone, add it to a 50ml single-necked round bottom flask, then add 5ml pyridine, 0.80mmol glutaric anhydride, add a stirrer, stir and react at room temperature for 10 hours; after the reaction, evaporate the solvent under reduced pressure , purified by thin-layer chromatography, TLC: the chromatographic solution is ethyl acetate, and the product ratio shift value Rf=0.2~0.3; the hapten (2) is obtained, and the specific synthetic route is as follows figure 1 shown.
[0067] (2) Preparation of ziprasidone artificial antigen
[0068] a. Weigh 0.10mmol of hapten in a 50ml round bottom flask, add 3ml of N,N-dimethylformamide (DMF), then add 0.28mmol of N-hydroxysuccinimide (NHS) and 0.48mmol of N,N-dimethylformamide Cyclohexylcarbodiimide (DCC), stirred overnight at room temperature, centrifuged after the reaction, and the supernatant was taken as liquid A.
[0069] b. Weigh 14.5 g of disodium hydrogen ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com