3D printing titanium interbody fusion cage and preparation method and application thereof
An intervertebral fusion device and 3D printing technology, applied in medical science, tissue regeneration, prosthesis, etc., can solve the problems of inability to reduce the incidence of postoperative infection and poor coating stability, and achieve the prevention of local postoperative infection, structure The effect of stabilizing and reducing the incidence of postoperative infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
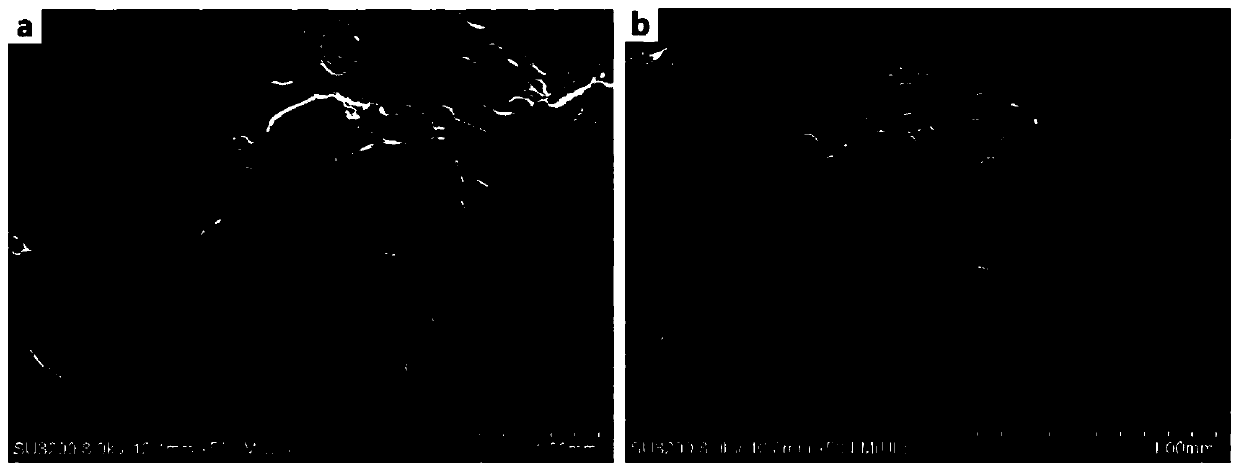
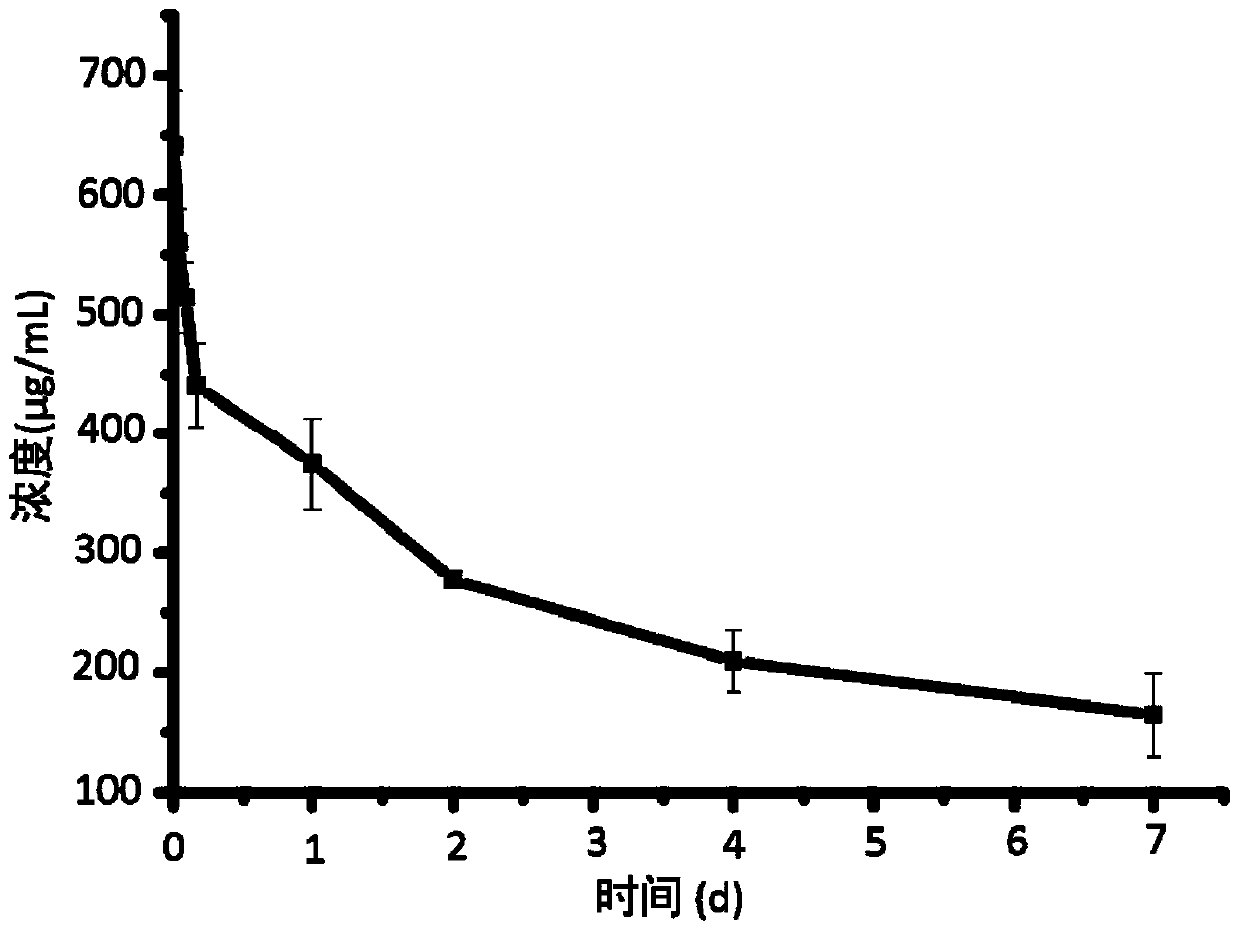
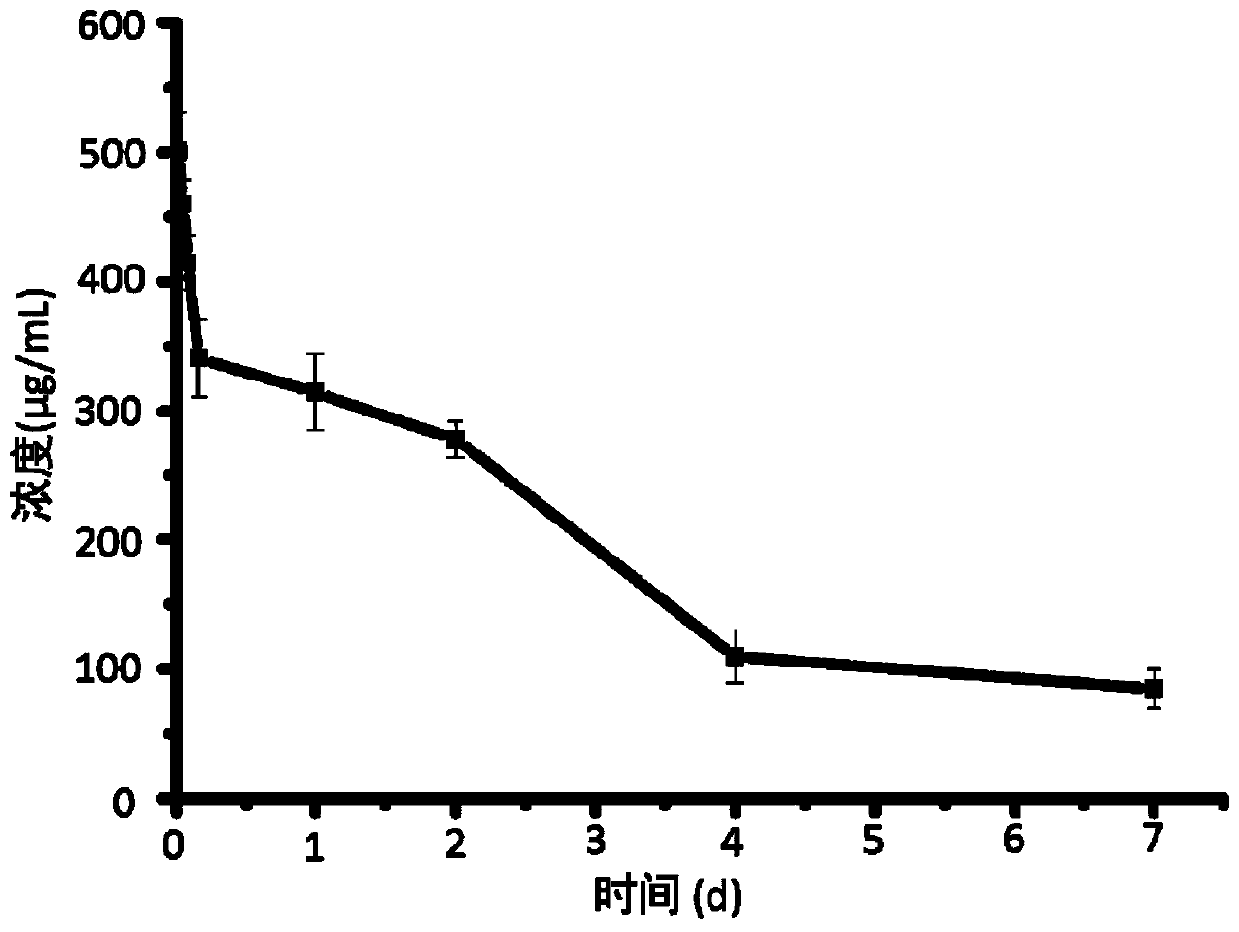
[0059] This embodiment provides a 3D printing titanium intervertebral fusion device, the 3D printing titanium intervertebral fusion device includes Ti 6 Al 4 V main structure and a drug coating coated on the surface of the main structure; the drug coating includes biodegradable polymer material polyvinyl alcohol (molecular weight 75000Da) and anti-infection drug vancomycin hydrochloride. The main body has a porous structure with an average pore diameter of 420 μm and a porosity of 70%. The mass ratio of polyvinyl alcohol to vancomycin hydrochloride is 3:1, and the load of the drug coating on the main structure is 1.0%.
[0060] Its preparation method comprises the following steps:
[0061] (1) The entity is designed by UG NX6.0 software, and the design model is obtained by 3-matic grid processing. 6 Al 4 V is the main material and is 3D printed with Acram Q10 equipment using metal electron beam melting technology. The electron beam output power is 3kW, the printing accurac...
Embodiment 2
[0066] This embodiment provides a 3D printing titanium intervertebral fusion device, the 3D printing titanium intervertebral fusion device includes Ti 6 Al 4 V main structure and a drug coating coated on the surface of the main structure; the drug coating includes biodegradable polymer material polylactic acid-glycolic acid copolymer (molecular weight 20000Da) and anti-infection drug levofloxacin hydrochloride. The main body has a porous structure with an average pore size of 400 μm and a porosity of 65%. Wherein the mass ratio of polylactic acid-glycolic acid copolymer to levofloxacin hydrochloride is 1:1, and the load of the drug coating on the main structure is 0.5%.
[0067] Its preparation method comprises the following steps:
[0068] (1) The entity is designed by UG NX6.0 software, and the design model is obtained by 3-matic grid processing. 6 Al 4 V is the main material and is 3D printed with Acram Q10 equipment using metal electron beam melting technology. The out...
Embodiment 3
[0073] This embodiment provides a 3D printing titanium intervertebral fusion device, the 3D printing titanium intervertebral fusion device includes Ti 6 Al 4 V main structure and a drug coating coated on the surface of the main structure; the drug coating includes biodegradable polymer material polyvinyl alcohol (molecular weight 79000Da) and anti-infection drug vancomycin hydrochloride. Wherein the mass ratio of polyvinyl alcohol to vancomycin hydrochloride is 5:1, and the load of the drug coating on the main structure is 1.5%.
[0074] Its preparation method comprises the following steps:
[0075] (1) The entity is designed by UG NX6.0 software, and the design model is obtained by 3-matic grid processing. 6 Al 4 V is the main material and is 3D printed with Acram Q10 equipment using metal electron beam melting technology. The output power of the electron beam is 2kW, the printing accuracy is ±0.4mm, and the layer thickness is 0.05mm. Ti 6 Al 4 V main structure;
[0076...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Layer thickness | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com