Dicofol hapten, artificial antigen and antibody, and synthesis method and application thereof
A technology for dicofol and its synthesis method, which is applied in the field of immunochemical analysis, can solve the problems of dicofol antibody specificity and sensitivity constraints, unsuitability for large-scale promotion and application, and relatively high accuracy requirements, and achieve easy control of reaction conditions and low synthesis cost Advantages, the effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
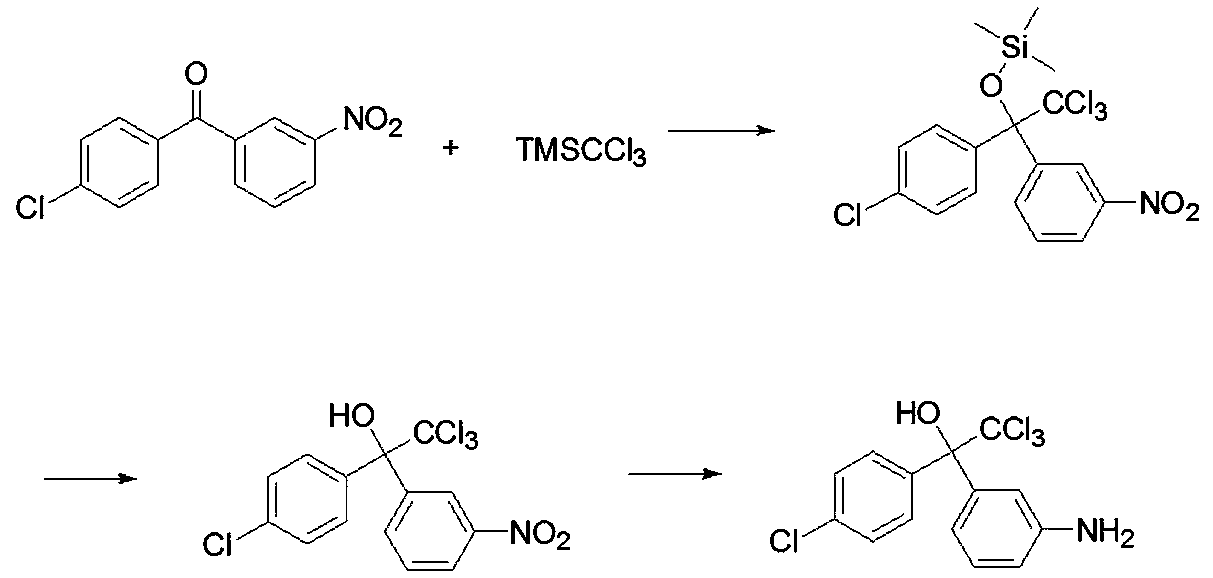
[0038] Specifically, the formula ( ) The synthetic method of the dicofol hapten of the structure shown comprises the following steps:
[0039] S1. Under the catalysis of the catalyst, (trichloromethyl)trimethylsilane (TMSCCl 3 ) reacts with 4-chloro-3-nitrobenzophenone to obtain intermediate 1, which has the structural formula ;
[0040] S2. The intermediate 1 is hydrolyzed to obtain the intermediate 2, and the intermediate 2 has the structural formula
[0041] ;
[0042] S3. The intermediate 2 is subjected to a reduction reaction to obtain the formula ( ) of the dicofol hapten of the structure shown.
[0043] Wherein, the step S1 comprises the following steps: first add 4-chloro-3-nitrobenzophenone, a catalyst dissolved in DMF to the reactor, and slowly add DMF-dissolved (trichloromethyl ) trimethylsilane, after the dropwise addition, the reaction was continuously stirred at room temperature. After the reaction, water was added, extracted with ethyl acetate, and th...
Embodiment 1
[0060] A kind of dicofol hapten, the steps of its synthetic method are as follows:
[0061] S1. First add 2.3g of 4-chloro-3-nitrobenzophenone and 0.23g of sodium formate dissolved in 30mL of DMF to the one-necked bottle, and then slowly add 2g of (trichloromethyl) trichloromethyl trichloride dissolved in DMF dropwise at room temperature. After the addition of methyl silane, continue stirring at room temperature for 2 hours. After the reaction, add water, extract 2-3 times with ethyl acetate, collect the organic phase, evaporate the organic phase to dryness, and then purify the column to obtain the intermediate 1, the intermediate 1 has the structural formula ;
[0062] S2. Weigh 2g of intermediate 1, dissolve it in 50mL of methanol, add 10mL of 20% hydrochloric acid solution, heat to reflux, and carry out the hydrolysis reaction for 15h; after the reaction, evaporate the methanol to dryness, add water to dilute, and then extract 2-3 times with ethyl acetate , the organic p...
Embodiment 2
[0065] A kind of dicofol hapten, the steps of its synthetic method are as follows:
[0066] S1. First add 2.3g of 4-chloro-3-nitrobenzophenone and 0.77g of ammonium acetate dissolved in 30mL of DMF to the one-mouth bottle, then slowly add 2g of (trichloromethyl) dissolved in DMF dropwise at room temperature After the dropwise addition of trimethylsilane, continue stirring at room temperature for 1 hour. After the reaction, add water, extract 2-3 times with ethyl acetate, collect the organic phase, evaporate the organic phase to dryness, and then pass column purification to obtain intermediate Body 1, said intermediate 1 has the structural formula ;
[0067]S2. Weigh 2g of intermediate 1, dissolve it with 50mL of methanol, add 10mL of 20% hydrobromic acid aqueous solution, heat to reflux, and carry out the hydrolysis reaction for 24h; The ester is extracted 2-3 times, and the organic phase is evaporated to dryness and purified by a column to obtain intermediate 2, which has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com