Green synthesis method of anticoccidial veterinary drug Ethanamizuril
A green synthesis and anti-coccidian technology, applied in the direction of organic chemistry, can solve the problems of not being suitable for industrial production, limiting the application prospect of drugs, complicated post-processing procedures, etc., and achieve the effects of shortening the overall reaction steps, low cost, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The green synthesis method of the anticoccidial veterinary drug samizuril comprises the following steps:
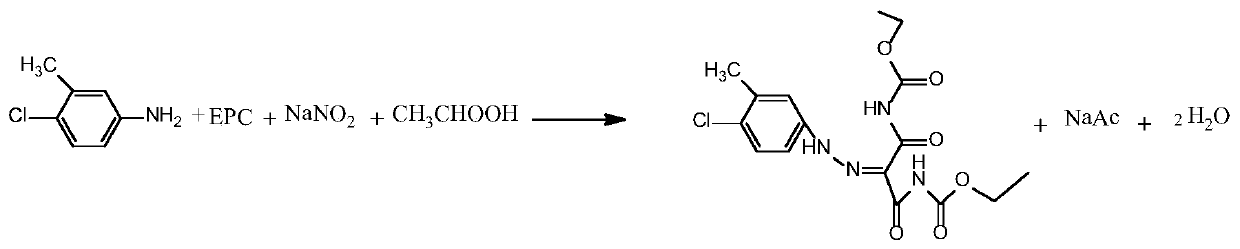
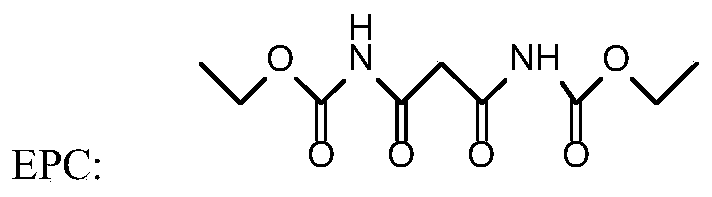
[0038] Such as figure 1 and figure 2 As shown, step 1 diazo coupling reaction: In a 1000ml four-necked flask, add 215g of carboxylic acid protic solvent acetic acid, 43g of 4-chloro-3-methylaniline, and 97.19g of diethoxycarbonylmalonamide (EPC) 125.71g of 20% sodium nitrite aqueous solution was added dropwise, and the mol ratio of 4-chloro-3-methylaniline, diethoxycarbonyl malonamide (EPC) and sodium nitrite was 1:1.3:1.2; the diazo coupling reaction temperature At 5°C, carry out the diazo coupling reaction, heat the reaction for 10 hours until the reaction is complete, and obtain the diazo coupling compound (structure 1);
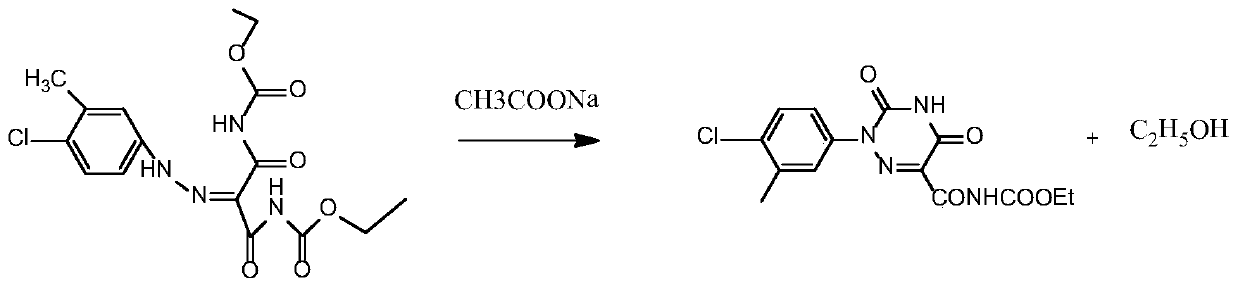
[0039] Such as image 3 As shown, the cyclization reaction of step 2: the diazo compound prepared in step 1 does not need to be separated, and 12.45 g of sodium acetate is directly added. The amount of sodium acetate added in the cyclizati...
Embodiment 2
[0044] The green synthesis method of the anticoccidial veterinary drug samizuril comprises the following steps:
[0045] Such as figure 1 and figure 2 As shown, step 1 diazo coupling reaction: In a 1000ml four-necked flask, add 215g of carboxylic acid protic solvent formic acid, 43g of 4-chloro-3-methylaniline, and 104.67g of diethoxycarbonyl malonamide (EPC) , drop 125.71g of 20% sodium nitrite aqueous solution, 4-chloro-3-methylaniline, diethoxycarbonyl malonamide (EPC) and sodium nitrite mol ratio are 1:1.4:1.2; Diazo coupling reaction temperature at 0°C, carry out the diazo coupling reaction, heat the reaction for 10 hours until the reaction is complete, and obtain the diazo coupling compound (structure 1);
[0046] Such as image 3 As shown, step 2 cyclization reaction: the diazo compound prepared in step 1 does not need to be separated, directly add 2.49 g of sodium acetate, the amount of sodium acetate added in the cyclization reaction and the 4-chloro-3 added in st...
Embodiment 3
[0051] The green synthesis method of the anticoccidial veterinary drug samizuril comprises the following steps:
[0052] Such as figure 1 and figure 2 As shown, step 1 diazo coupling reaction: In a 1000ml four-necked flask, add 215g of carboxylic acid protic solvent propionic acid, 43g of 4-chloro-3-methylaniline, 119.62g of diethoxycarbonyl malonamide (EPC) g. Add 157.14 g of 20% sodium nitrite aqueous solution dropwise, the molar ratio of 4-chloro-3-methylaniline, diethoxycarbonyl malonamide (EPC) and sodium nitrite is 1:1.6:1.5; diazo coupling reaction The temperature is 30°C, and the diazo coupling reaction is carried out, and the reaction is kept for 5 hours until the reaction is complete, and the diazo coupling compound (structure 1) is obtained;
[0053] Such as image 3 As shown, the cyclization reaction of step 2: the diazo compound prepared in step 1 does not need to be separated, and 12.45 g of sodium acetate is directly added. The amount of sodium acetate added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com