RAA amplification primer and probe for rapid detection of Cyprinid herpesvirus type II, detection kit and application method thereof
A technology of carp herpes virus and amplification primers, which is applied in the field of RAA amplification primers, probes and detection kits for rapid detection of carp herpes virus type II, and can solve the problems of immunohistochemical methods that need to be verified and virus cell lines that need to be improved, etc. problem, to achieve the effect of rapid response, high specificity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The establishment of the RAA-LFD rapid detection kit of embodiment 1CyHV-2
[0036] 1. Design and synthesis of RAA primers and probes for CyHV-2
[0037] Taking the conserved region of the helicase gene of CyHV-2 in the NCBI database as the target site, according to the principle of RAA primer design, DNAman 6.0 software was used for sequence alignment analysis, and fragments with high homology were selected, and 4 pairs of primers were designed with primer primer 5.0 (as in Table 1).
[0038] Table 1 Primers used in RAA
[0039]
[0040] 2. CyHV-2RAA detection primer screening
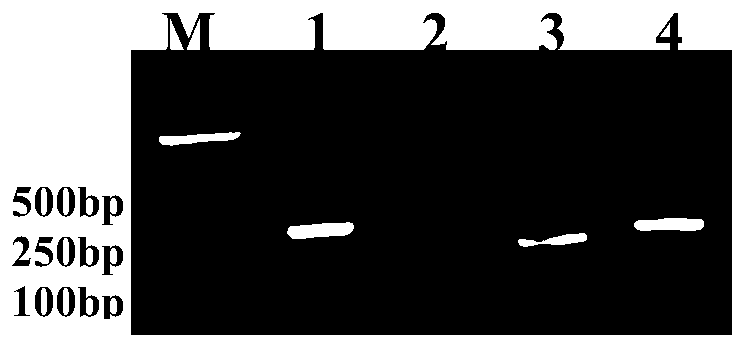
[0041] With CyHV-2 positive plasmid (8.14×10 6 copies / μL) as the template for amplification, after the reaction, use 2% agarose electrophoresis to identify (see figure 1 ), screen the designed primers, and screen out the optimal primer pair as the third group of primers, and the optimal primer pair is F and R:
[0042] Upstream primers:
[0043] RAA-GFHNV-3-F: 5'-ATCTTCAAGCCCAAGATCAATC...
Embodiment 3
[0063] The sensitivity test of embodiment 3 kits of the present invention
[0064] The CyHV-2 positive plasmid standard substance that kit described in the embodiment of the present invention 1 provides, measures concentration with NanoDrop, according to formula﹛plasmid concentration (ng / μL) * 10 -9 / [660×(plasmid length+vector length)]﹜×6.023×10 23 = Plasmid copy number (copies / μL) converted to its copy number. The plasmids were divided into 8.14×10 8 to 8.14×10 0 Copy / μL 9 concentration gradient dilutions were used for sensitivity test.
[0065] Test results such as figure 2 As shown, except the negative control, from left to right is 8.14×10 0 ~8.14×10 8 The amplification result of the positive standard product of copy / μL, can find out that the RAA-LFD sensitivity minimum detection limit of the present invention 8.14 * 10 0 copy / μL, the sensitivity is better than that of ordinary PCR detection method, which shows that the RAA-LFD rapid detection kit and detection me...
Embodiment 4
[0066] The specificity test of embodiment 4 kits of the present invention
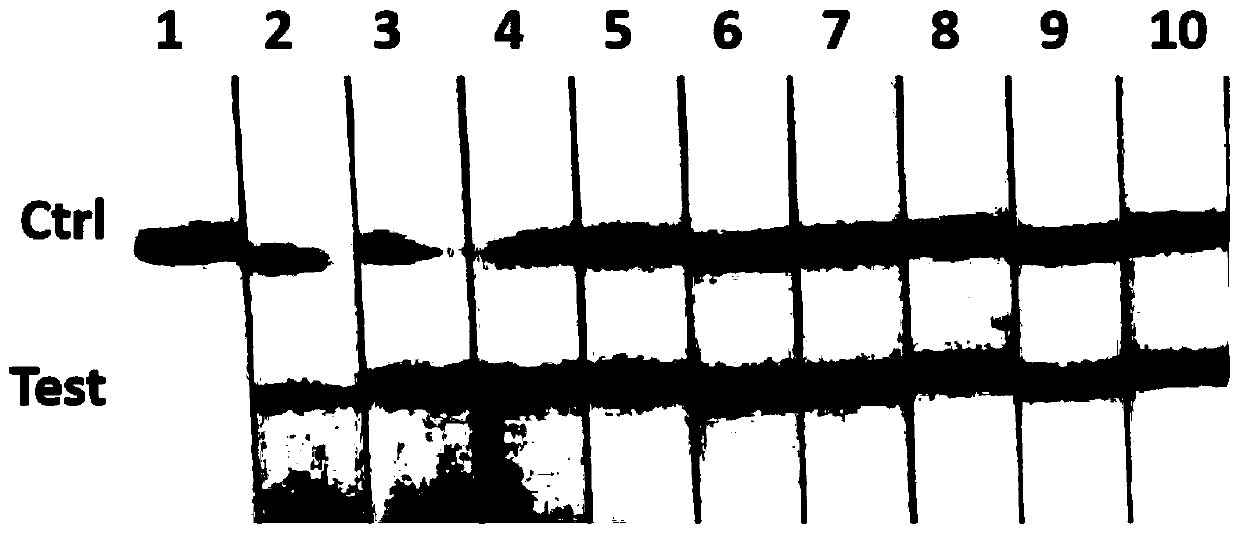
[0067] In order to detect the specificity of the kit of the present invention, the detection method in Example 2 is used to detect the positive samples of viruses CyHV-2, IHNV, SVCV, GV, CEV, KHV, EHNV, CCV respectively, and analyze the effect of the kit on CyHV -2 and other common DNA viruses in aquatic animals.
[0068] The test results showed that only the CEV samples had normal amplification, and the negative control (DEPC-treated water) and KHV, GFHNV, EHNV, BIV, CCV, WSSV, and EHP samples had no amplification (such as image 3 shown). The above results indicate that the RAA-LFD rapid detection kit of the present invention can specifically amplify the target sequence in CEV without cross-reaction with other viral nucleic acids. It shows that the method and kit of the present invention have good specificity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com