Quenching modification method for improving electrocatalytic property of metal oxide, prepared metal oxide electrocatalyst and application
A technology for the electro-catalytic performance of oxides, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, fuel cell-type half-cells and primary battery-type half-cells, etc. Catalytic performance improvement, complex synthesis steps, resource consumption and other problems, to achieve the effect of improving electrocatalytic activity, good ORR performance, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
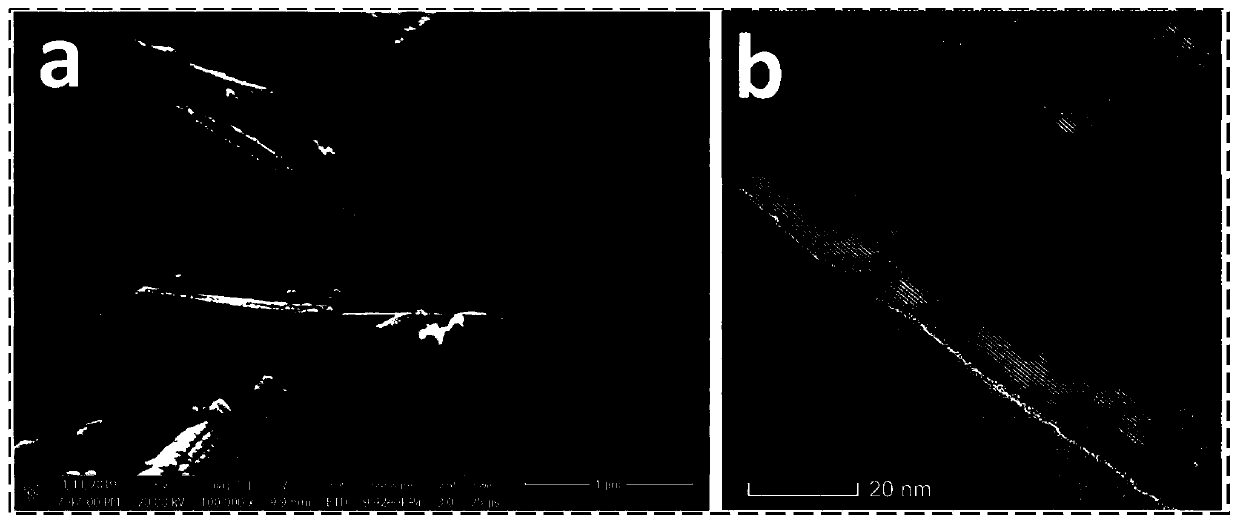
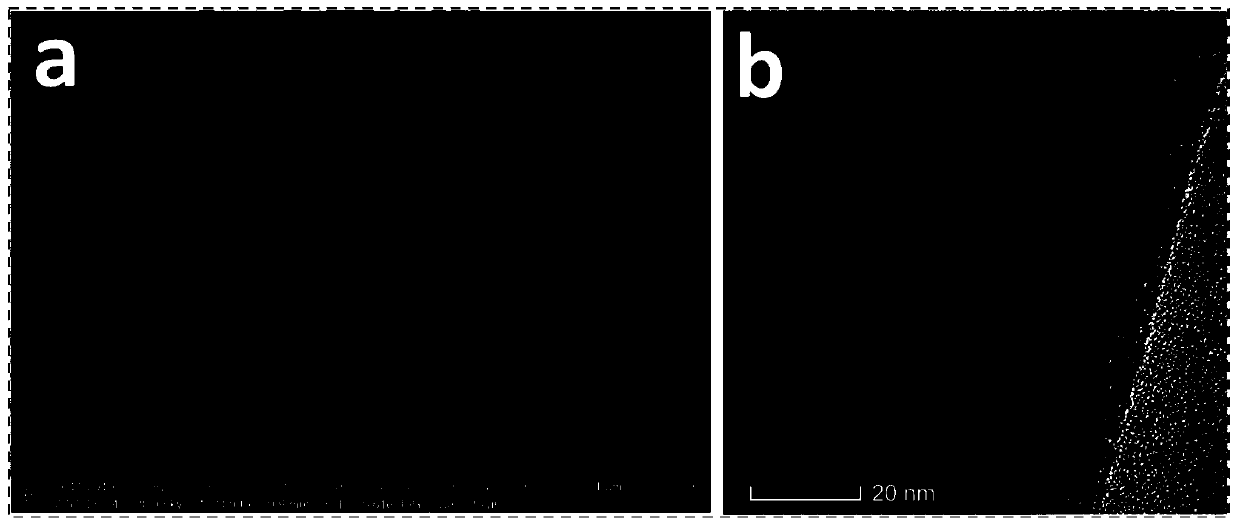
Image
Examples
Embodiment 1
[0071] (1) Add 2.622 g ammonium molybdate, 4.362 g nickel nitrate and 0.45 g urea into a 100 ml reaction kettle, then add 70 ml deionized water, stir for 30 minutes, and then react at 160 °C for 12 h. After cooling, the powder was separated with a centrifuge, washed alternately with deionized water and ethanol, and then dried under vacuum at 60 °C to obtain NiMoO 4 Precursor.
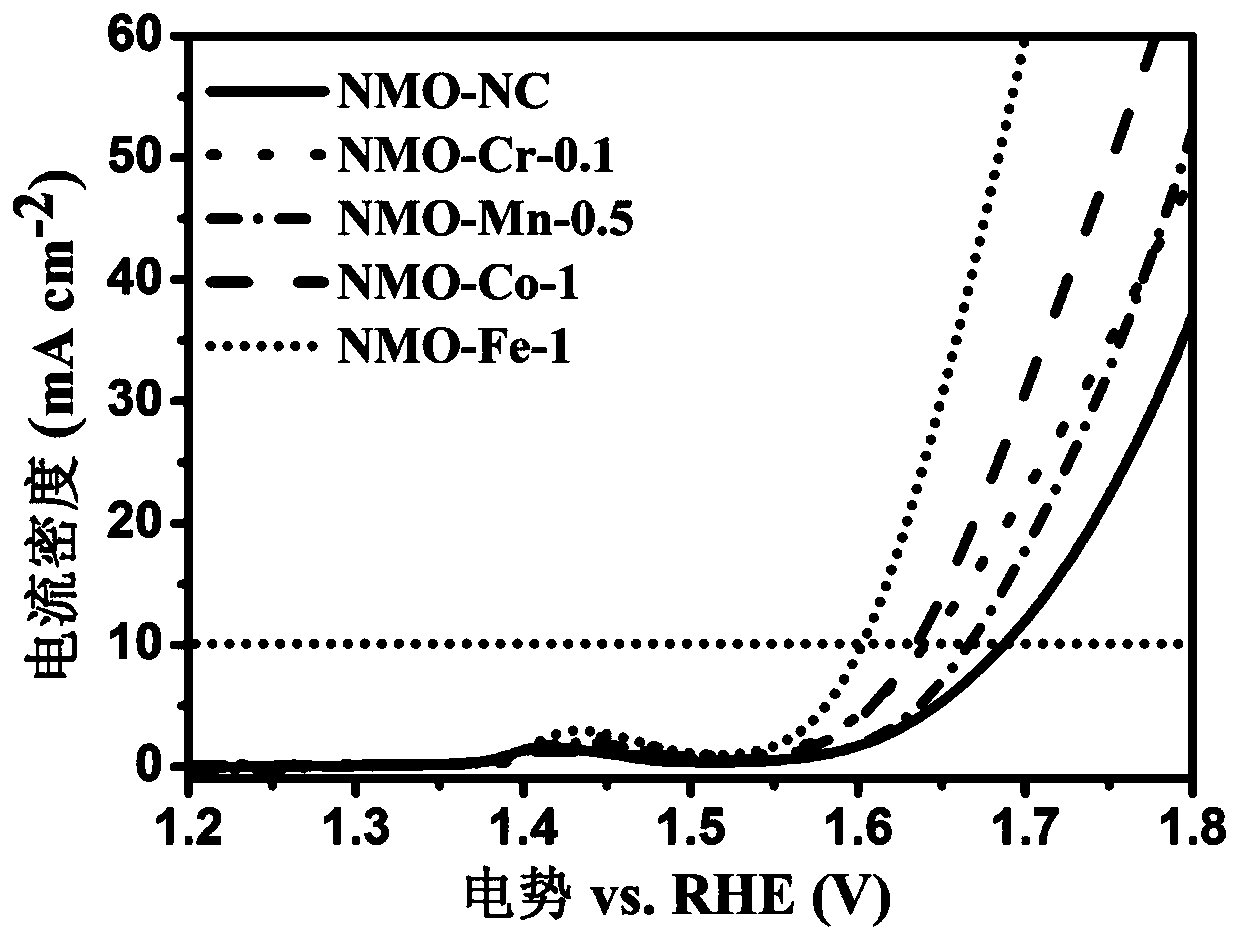
[0072] (2) Take 200 mg NiMoO 4 The precursor was placed in a muffle furnace, and the temperature was raised to 500 °C at a heating rate of 5 °C / min and kept constant for 2 h, then the powder was quickly taken out and placed in a container containing 1 M Fe(NO 3 ) 3 In the ice water solution, the Fe(NO 3 ) 3 The temperature of the ice-water solution was 0 °C and stirred for 1 h at a stirring rate of 700 rpm. Suction filtration was then performed to separate the powder, washed with a large amount of deionized water, and finally air-dried at 60°C, and recorded as NMO-Fe-1.
[0073] (3) The working el...
Embodiment 2
[0076] (1) Add 2.622 g ammonium molybdate, 4.362 g nickel nitrate and 0.45 g urea into a 100 ml reaction kettle, then add 70 ml deionized water, stir for 30 minutes, and then react at 160 °C for 12 h. After cooling, the powder was separated with a centrifuge, washed alternately with deionized water and ethanol, and then dried under vacuum at 60 °C to obtain NiMoO 4 Precursor.
[0077] (2) Take 200 mg NiMoO 4 The precursor was placed in a muffle furnace, and the temperature was raised to 500 °C at a heating rate of 5 °C / min and kept constant for 2 h, then the powder was quickly taken out and placed in a container containing 1 M Co(NO 3 ) 2 In the ice water solution, the Co(NO3 ) 2 The temperature of the ice-water solution was 0 °C and stirred for 1 h at a stirring speed of 700 rpm. Suction filtration was then performed to separate the powder, washed with a large amount of deionized water, and finally air-dried at 60°C, and recorded as NMO-Co-1.
[0078] (3) The working ele...
Embodiment 3
[0080] (1) Add 2.622 g ammonium molybdate, 4.362 g nickel nitrate and 0.45 g urea into a 100 ml reaction kettle, then add 70 ml deionized water, stir for 30 minutes, and then react at 160 °C for 12 h. After cooling, the powder was separated with a centrifuge, washed alternately with deionized water and ethanol, and then dried under vacuum at 60 °C to obtain NiMoO 4 Precursor.
[0081] (2) Take 200 mg NiMoO 4 The precursor was placed in a muffle furnace, and the temperature was raised to 500 °C at a heating rate of 5 °C / min and kept constant for 2 h, then the powder was quickly taken out, and placed in a 0.1 M Cr(NO 3 ) 3 In the ice water solution, the Cr(NO 3 ) 3 The temperature of the ice-water solution was 0 °C and stirred for 1 h at a stirring rate of 700 rpm. Suction filtration was then performed to separate the powder, washed with a large amount of deionized water, and finally air-dried at 60°C, and recorded as NMO-Cr-0.1.
[0082] (3) The working electrode was prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com