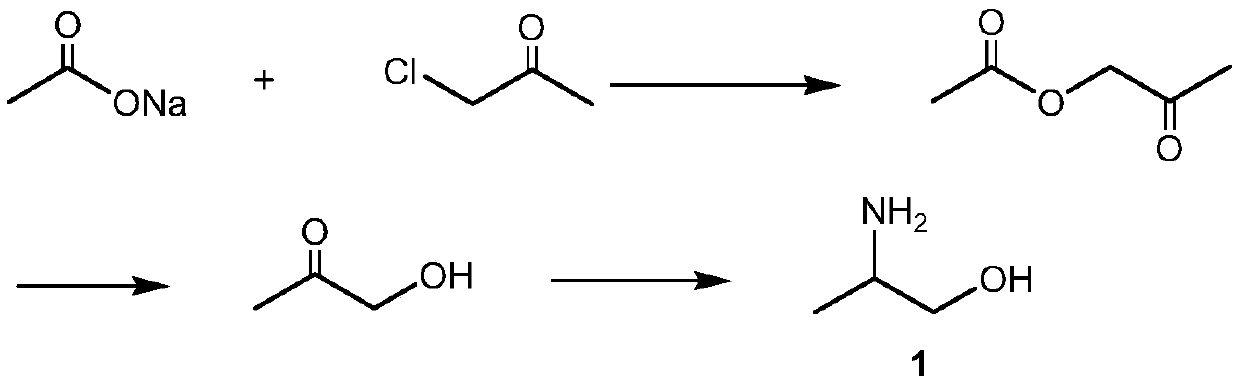
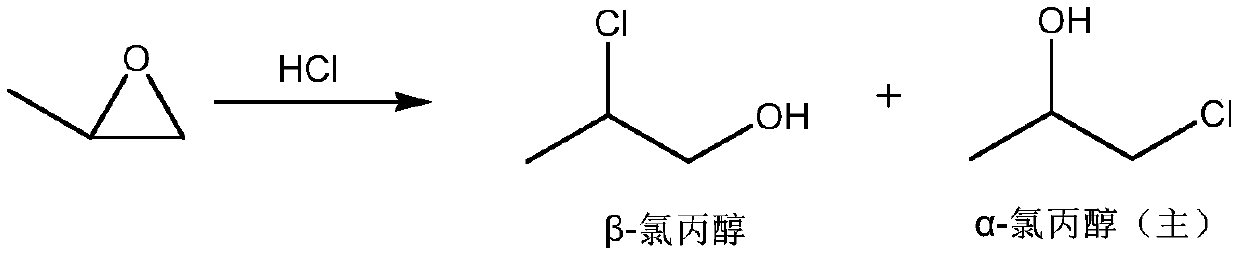
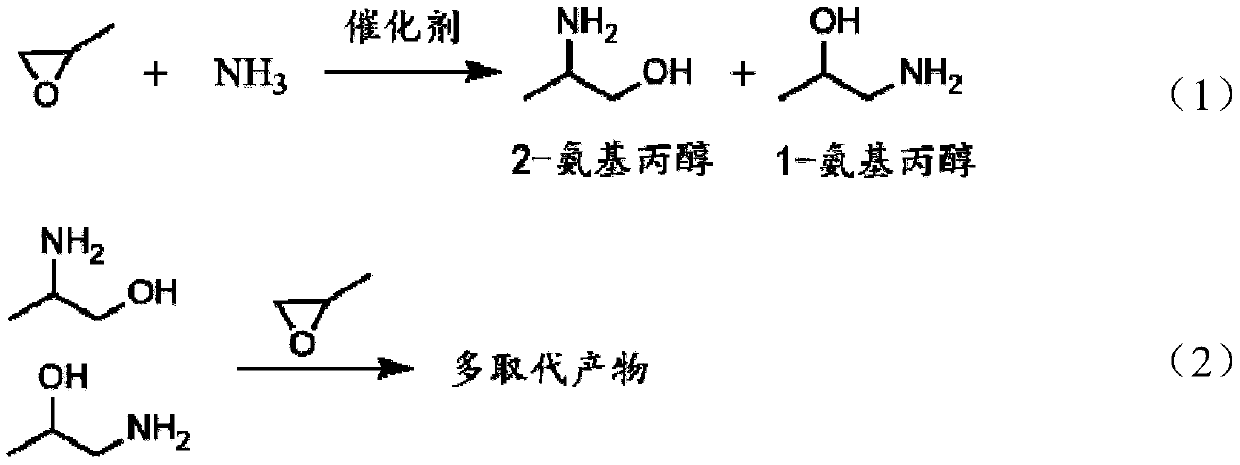
Synthesis method of 2-aminopropanol
A synthesis method and aminopropanol technology are applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of high production cost, high risk, and many by-products, and achieve convenient operation, The effect of less by-product formation and emission reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Rare Earth Modified Catalyst The hydrogen-type mordenite molecular sieve with a silicon-aluminum ratio of 22:1 and 0.3mol / L cerium nitrate were ion-exchanged at 80°C for 4 hours at a solid-to-liquid mass ratio of 1:15, and then washed, Filtrate and dry, and program the temperature to 550° C. to roast for 4 hours to obtain a cerium-modified hydrogen-type mordenite (H-MOR) molecular sieve catalyst.
[0035] Preparation of 2-aminopropanol In a tubular fixed-bed reactor, place a cerium-modified catalyst, raise the temperature of the system to 150°C, and then mix and pass it into the Stay in a tubular fixed-bed reactor for 30 seconds to obtain 2-aminopropanol.
Embodiment 2
[0037] Preparation of Rare Earth Modified Catalyst The hydrogen-type mordenite molecular sieve with a silicon-aluminum ratio of 22:1 and 0.3mol / L lanthanum nitrate were ion-exchanged at 80°C for 4 hours at a solid-to-liquid mass ratio of 1:15, and then washed, Filtrate and dry, and program the temperature to 550° C. to roast for 4 hours to obtain a lanthanum-modified hydrogen-type mordenite (H-MOR) molecular sieve catalyst.
[0038] Preparation of 2-aminopropanol In a tubular fixed-bed reactor, place a lanthanum-modified catalyst, raise the temperature of the system to 150°C, and then mix and pass it into the Stay in a tubular fixed-bed reactor for 30 seconds to obtain 2-aminopropanol.
Embodiment 3
[0040] Preparation of Rare Earth Modified Catalyst The hydrogen-type mordenite molecular sieve with a silicon-aluminum ratio of 22:1 and 0.3mol / L yttrium nitrate were ion-exchanged at 80°C for 4 hours at a solid-to-liquid mass ratio of 1:15, and then washed, After filtering and drying, the temperature was programmed to rise to 550° C. and calcined for 4 hours to obtain a yttrium-modified hydrogen-type mordenite (H-MOR) molecular sieve catalyst.
[0041] Preparation of 2-aminopropanol In a tubular fixed-bed reactor, a yttrium-modified catalyst was placed, the system was heated up to 150°C, and then mixed and passed into the Stay in a tubular fixed-bed reactor for 30 seconds to obtain 2-aminopropanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com