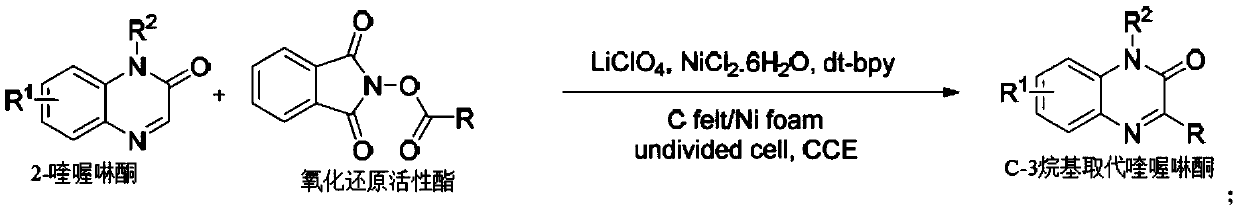
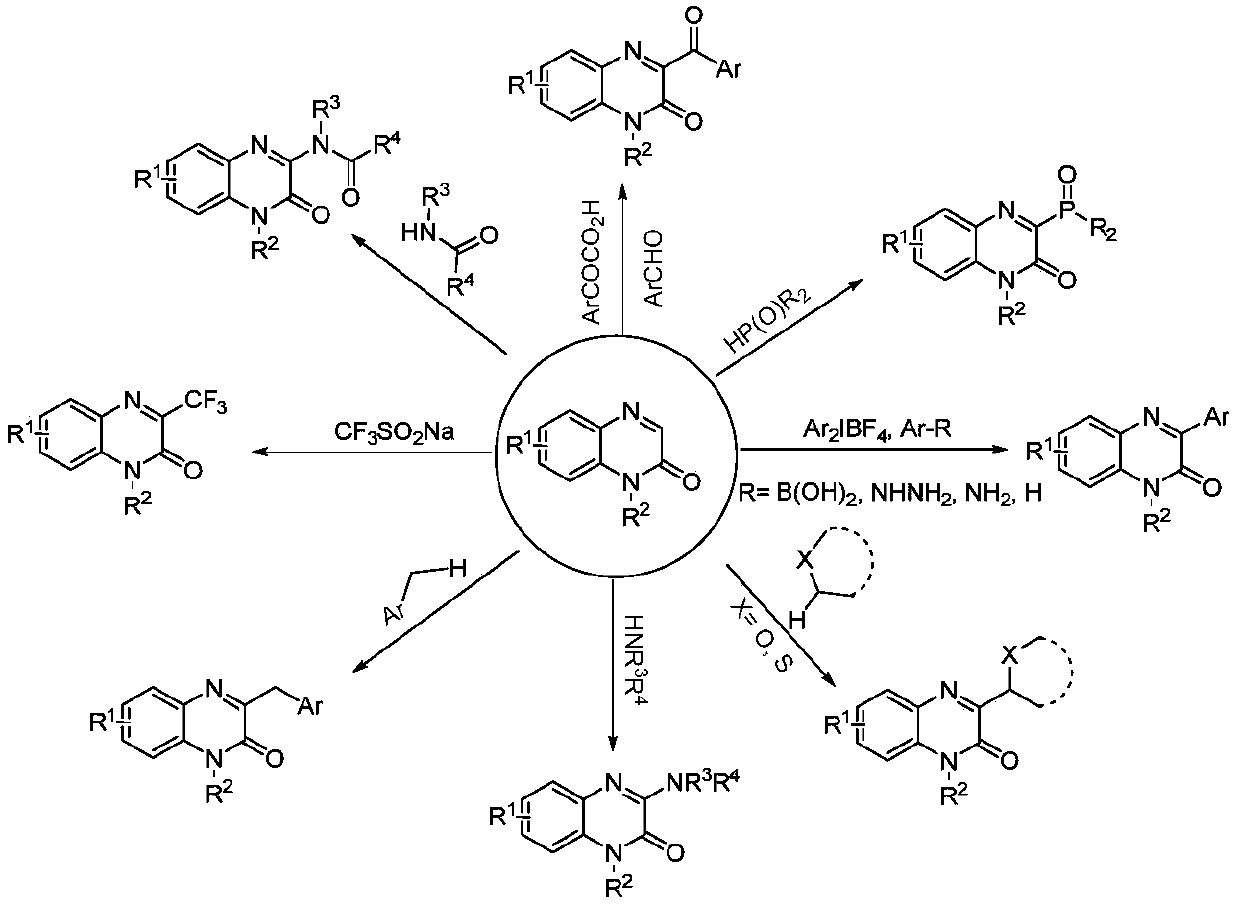
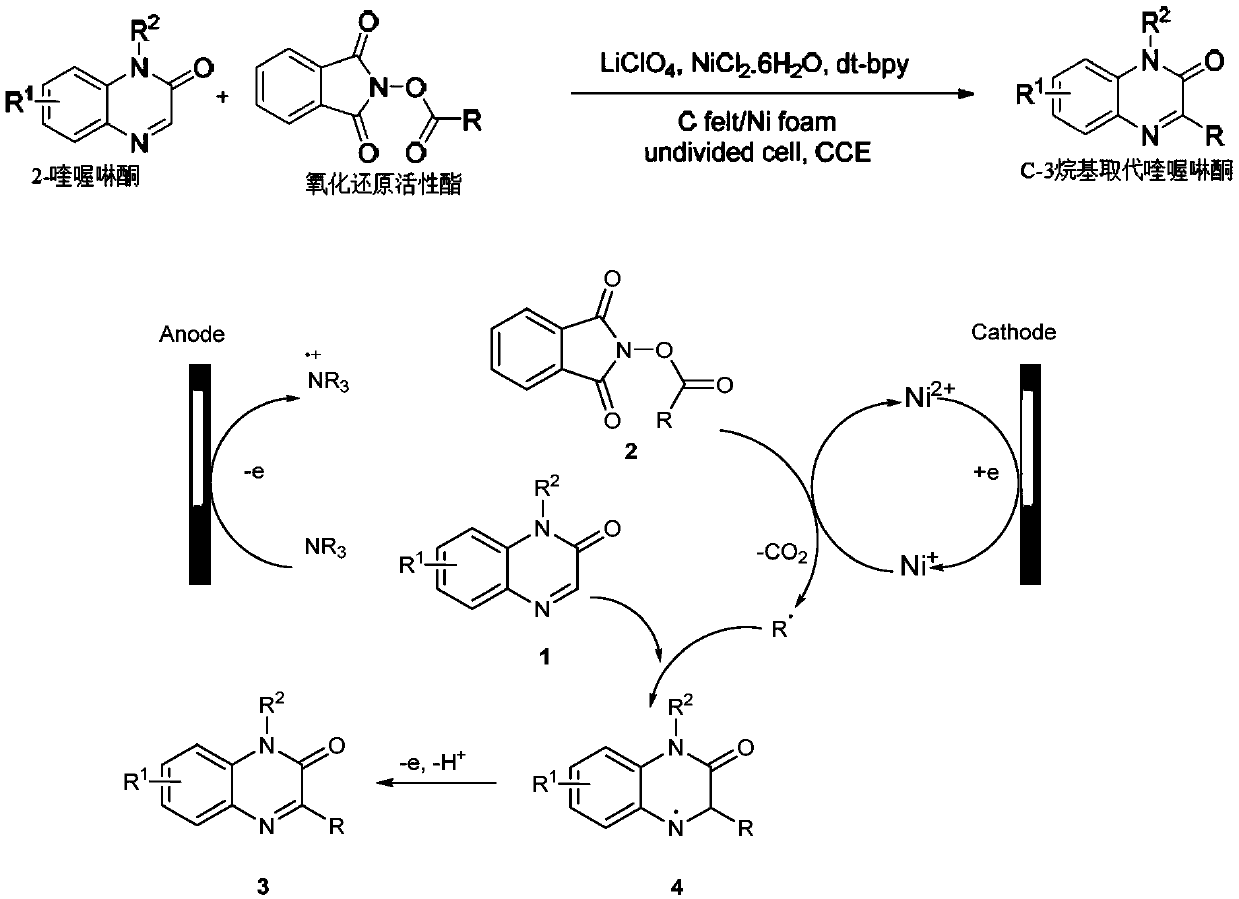
Method for synthesizing C-3 alkyl substituted quinoxalinone by nickel catalysis under an electrochemical condition
A quinoxalinone and synthesis method technology, applied in the direction of electrolysis components, electrodes, electrolysis process, etc., can solve the problems of low yield, high reaction temperature, environmental pollution, etc., and achieve the goal of reducing energy consumption, simple operation, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Electrochemical method synthesizes 3-cyclohexyl quinoline-2 (1H)-ketone
[0024] In a 10ml single-chamber electrolytic cell, add raw material 2-quinoxalinone (0.3mmol), redox active ester (0.6mmol), LiClO 4 (1.0mmol), NiCl 2 .6H 2 O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bipyridine (0.6 mmol). The apparatus was sealed and argon was injected into the tube (three times). Then, under an argon atmosphere, N,N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe and closed with a rubber stopper, and an argon-filled balloon was inserted into the bottle. The mixture was first reacted under magnetic stirring at 60°C for 30 minutes, and then 2 Electrolyze for 3 hours at current density. After the reaction was complete, the mixture was quenched with water and extracted with ethyl acetate (3 x 10ml). The organic phase was concentrated on a rotary evaporator. The desired product was purified by column chromatography in a silica gel ...
Embodiment 2
[0027] Embodiment 2: Electrochemical synthesis of 3-(4-isopropylcyclohexyl)quinoxalin-2(1H)-one
[0028] In a 10ml single-chamber electrolytic cell, add raw material 2-quinoxalinone (0.3mmol), redox active ester (0.6mmol), LiClO 4 (1.0mmol), NiCl 2 .6H 2 O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bipyridine (0.6 mmol). The apparatus was sealed and argon was injected into the tube (three times). Then, under an argon atmosphere, N,N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe and closed with a rubber stopper, and an argon-filled balloon was inserted into the bottle. The mixture was first reacted under magnetic stirring at 60°C for 30 minutes, and then 2Electrolyze for 3 hours at current density. After the reaction was complete, the mixture was quenched with water and extracted with ethyl acetate (3 x 10ml). The organic phase was concentrated on a rotary evaporator. The desired product was purified by column chromatography on silica gel ...
Embodiment 3
[0031] Example 3: Electrochemical synthesis of 3-(4,4-difluorocyclohexyl)quinoxalin-2(1H)-one
[0032] In a 10ml single-chamber electrolytic cell, add raw material 2-quinoxalinone (0.3mmol), redox active ester (0.6mmol), LiClO 4 (1.0mmol), NiCl 2 .6H 2 O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bipyridine (0.6 mmol). The apparatus was sealed and argon was injected into the tube (three times). Then, under an argon atmosphere, N,N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe and closed with a rubber stopper, and an argon-filled balloon was inserted into the bottle. The mixture was first reacted under magnetic stirring at 60°C for 30 minutes, and then 2 Electrolyze for 3 hours at current density. After the reaction was complete, the mixture was quenched with water and extracted with ethyl acetate (3 x 10ml). The organic phase was concentrated on a rotary evaporator. The desired product was purified by column chromatography on silica gel (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com