Preparation method of slow-release drug-loaded porous membrane based on supercritical fluid technology and apparatus thereof
A technology of supercritical fluid and porous membrane, which is applied in separation methods, pharmaceutical formulations, chemical instruments and methods, etc. It can solve the problems of difficult control of the preparation process, influence on the stability of the preparation process, and unstable structure and performance of the drug-loaded membrane. Effective treatment concentration, shorten drying time, ensure the effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
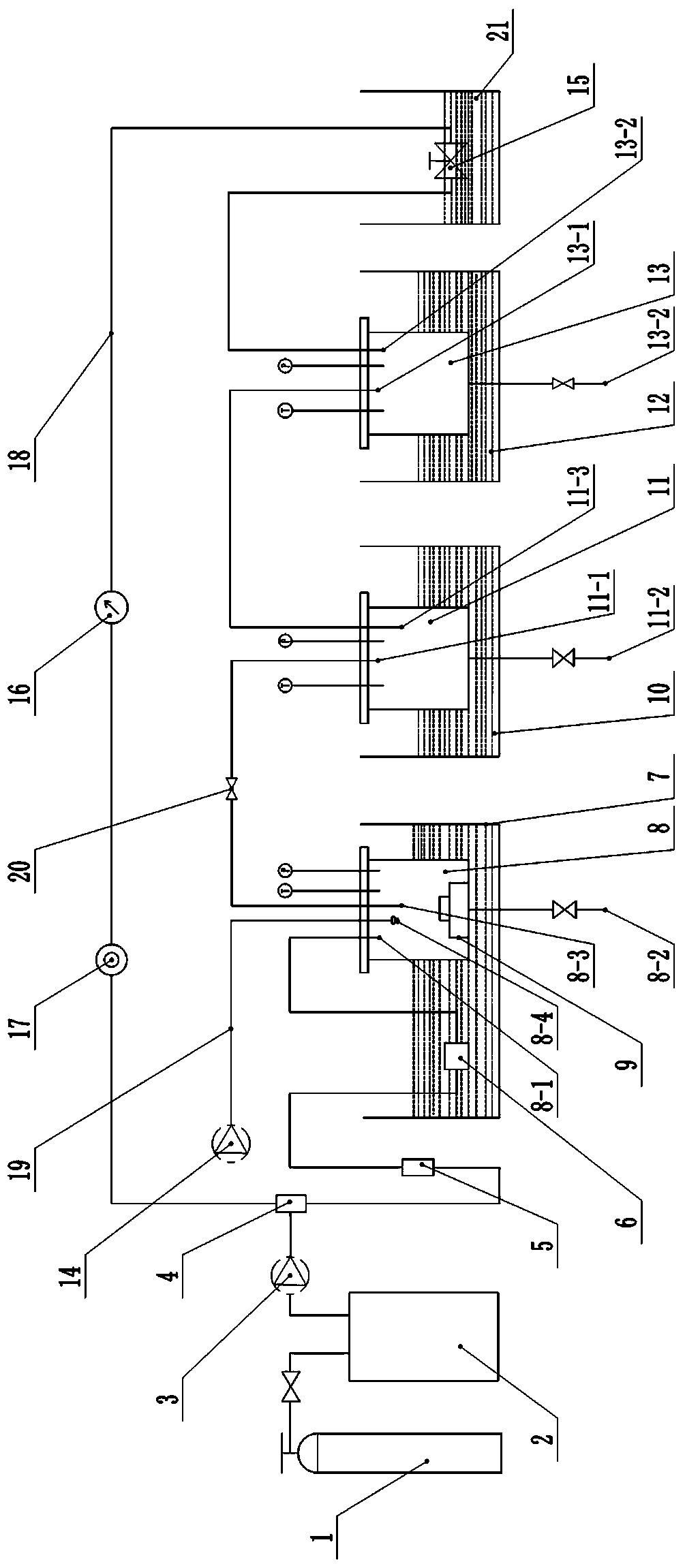
[0042] Such as Figure 1~3 As shown, a drug-loaded porous film preparation device used in the above preparation method, which includes CO connected sequentially through the main pipeline 2 Gas cylinder 1, cooling water tank 2, plunger pump 3, mixer 4, purifier 5, preheater 6, autoclave 8, primary separation kettle 11 and secondary separation kettle 13.
[0043] A reflux pipe 18 is arranged between the secondary separator 13 and the mixer 4, and a reflux valve 15, a flow meter 16 and a check valve 17 are arranged in sequence on the said reflux pipe 18; The branch pipeline 19 is provided with an advection pump 14 at the end of the branch pipeline 19.
[0044] The backflow valve 15 is arranged in the anti-clogging water bath device 21 . The anti-clogging water bath device can be a simple device, that is, the backflow valve is placed in a water bath with a temperature of 50-60° C., and the blocking rate of the backflow valve will be greatly reduced. Because the temperature is su...
Embodiment 2
[0052] A method for preparing a drug-loaded porous film with sustained release based on supercritical fluid technology using the device described in Example 1, comprising the following steps:
[0053] A. Weigh a certain mass of dry polymer, or a mixture of polymers with different mechanical properties and a certain mass of solid drug, and then dissolve the above polymer and drug into the solvent at the same time to make a concentration of 15% by mass. %-50% solution or suspension, after the solution or suspension is prepared, let it stand for 5-30min to remove the air bubbles.
[0054] Among them, the polymer can be but not limited to polymethylmethacrylate, polylactic acid, polycaprolactone or a mixture of polylactic acid and polycaprolactone. The solid drug can be but not limited to erythromycin, griseofulvin and other toxic antibiotic microparticles or nucleic acid, protein and polypeptide biochemical macromolecular drug microparticles. The solvent can be acetone, ethanol,...
Embodiment 3
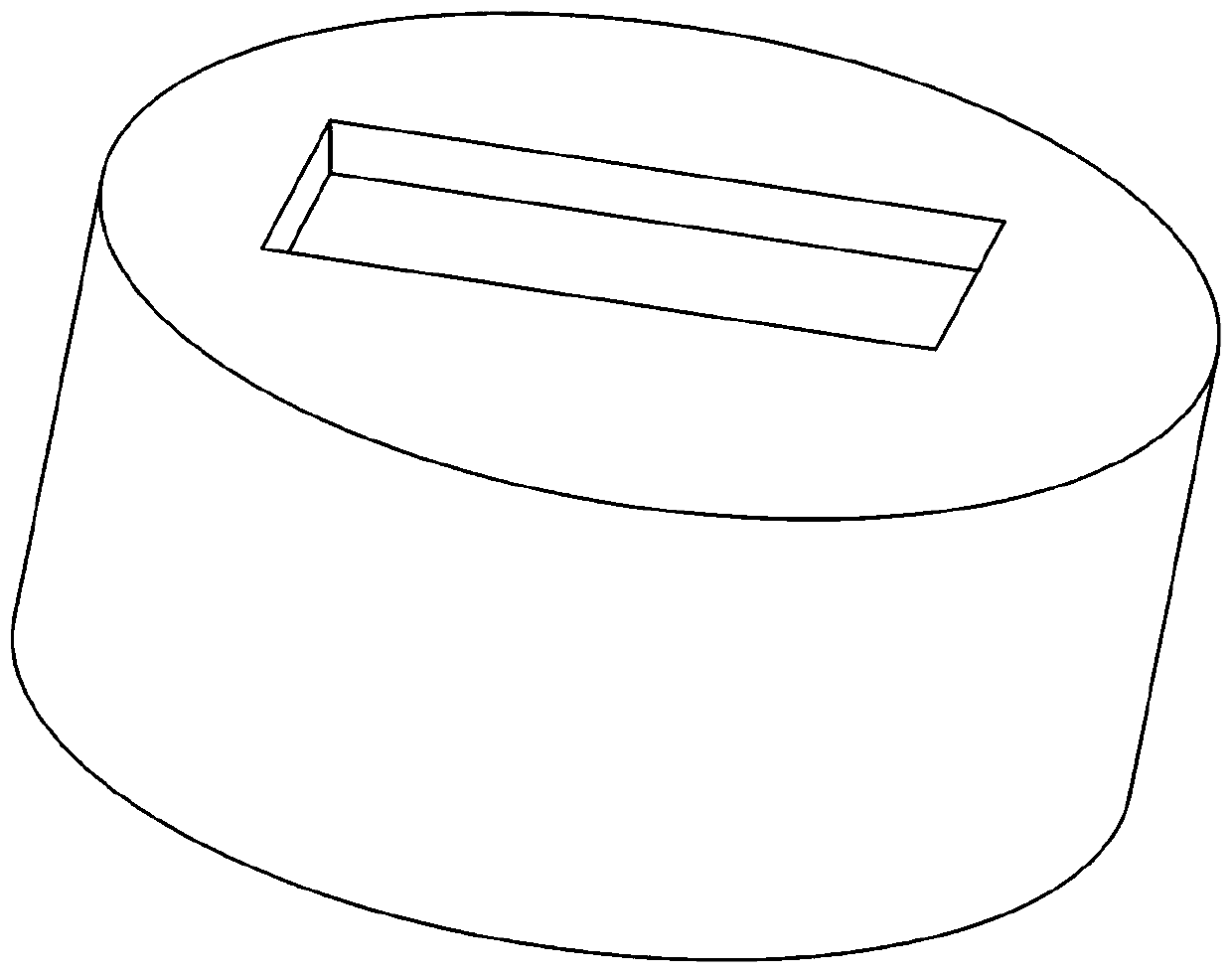
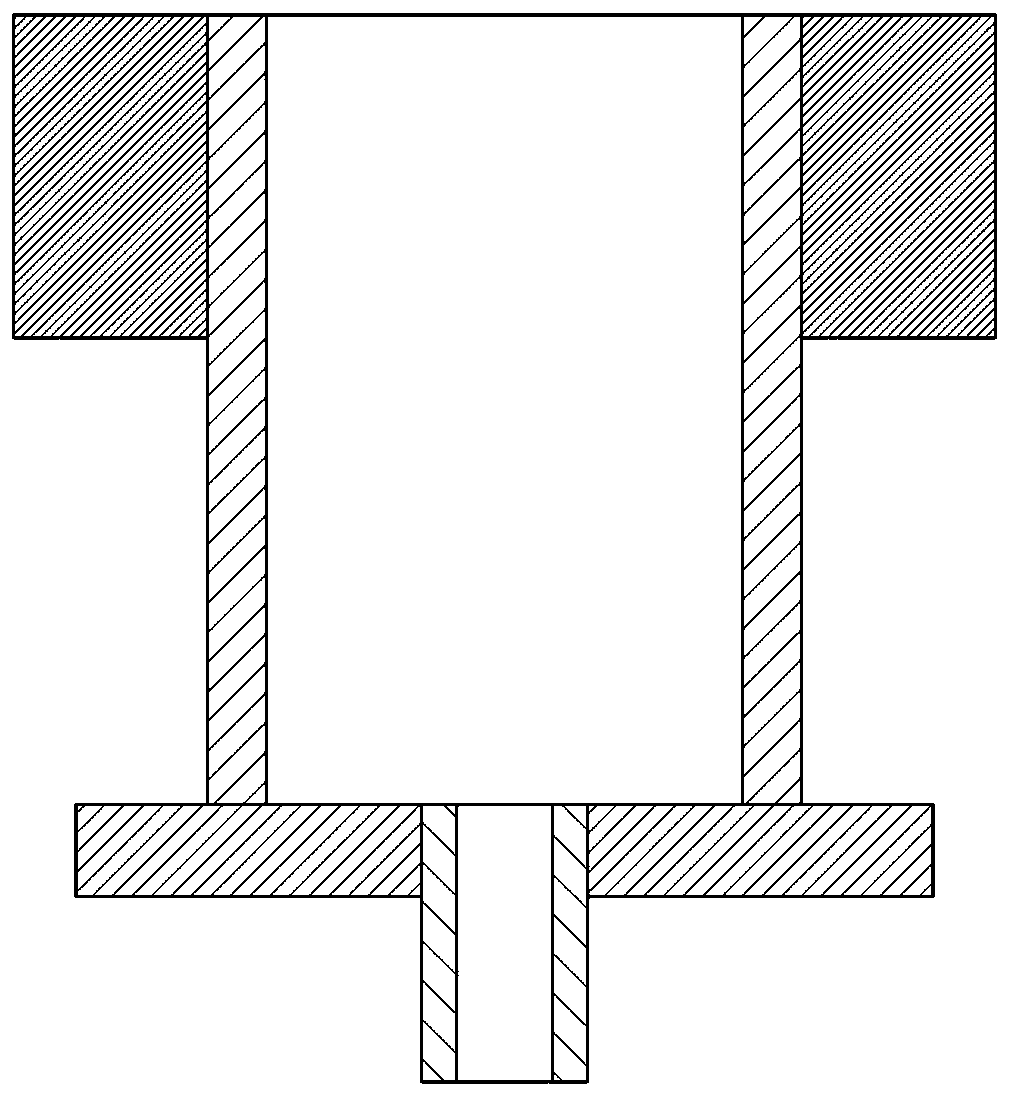
[0062] First, weigh 5 g of polylactic acid polymer and 2 g of 5-fluorouracil drug, add the above two solutes into acetone with a volume of 34 ml at the same time, and prepare a suspension with a mass percentage concentration of polylactic acid of about 15% by stirring. The solution is then poured into an aluminum film caster with a height of 1.5 cm and a diameter of 4 cm, the upper surface of which has a 0.1-0.5 mm thick rectangular groove. Use a spatula to spread it into a uniform thin layer with a certain thickness, and then quickly put the film caster into an autoclave at 35°C. Turn on the CO 2 Gas cylinder and plunger pump, blowing CO into the autoclave 2 , start the advection pump at the same time, spray absolute ethanol as a carrier agent into the autoclave through the nozzle, and when the pressure of the autoclave reaches 15MPa, turn off the advection pump, close the inlet and outlet valves of the autoclave, and keep the pressure. After 60min, open the inlet and outle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| Depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com