Pharmaceutical composition containing tea leaf extract and application of pharmaceutical composition in treatment of cancers
A technology of tea extract and composition, which is applied in the field of medicine composition containing tea extract to treat cancer, can solve the problems of not being able to cure lung cancer, blindly exaggerate the therapeutic effect, and dismiss it, so as to achieve the recovery of mental state and physical strength , reduce toxic and side effects, and prolong survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
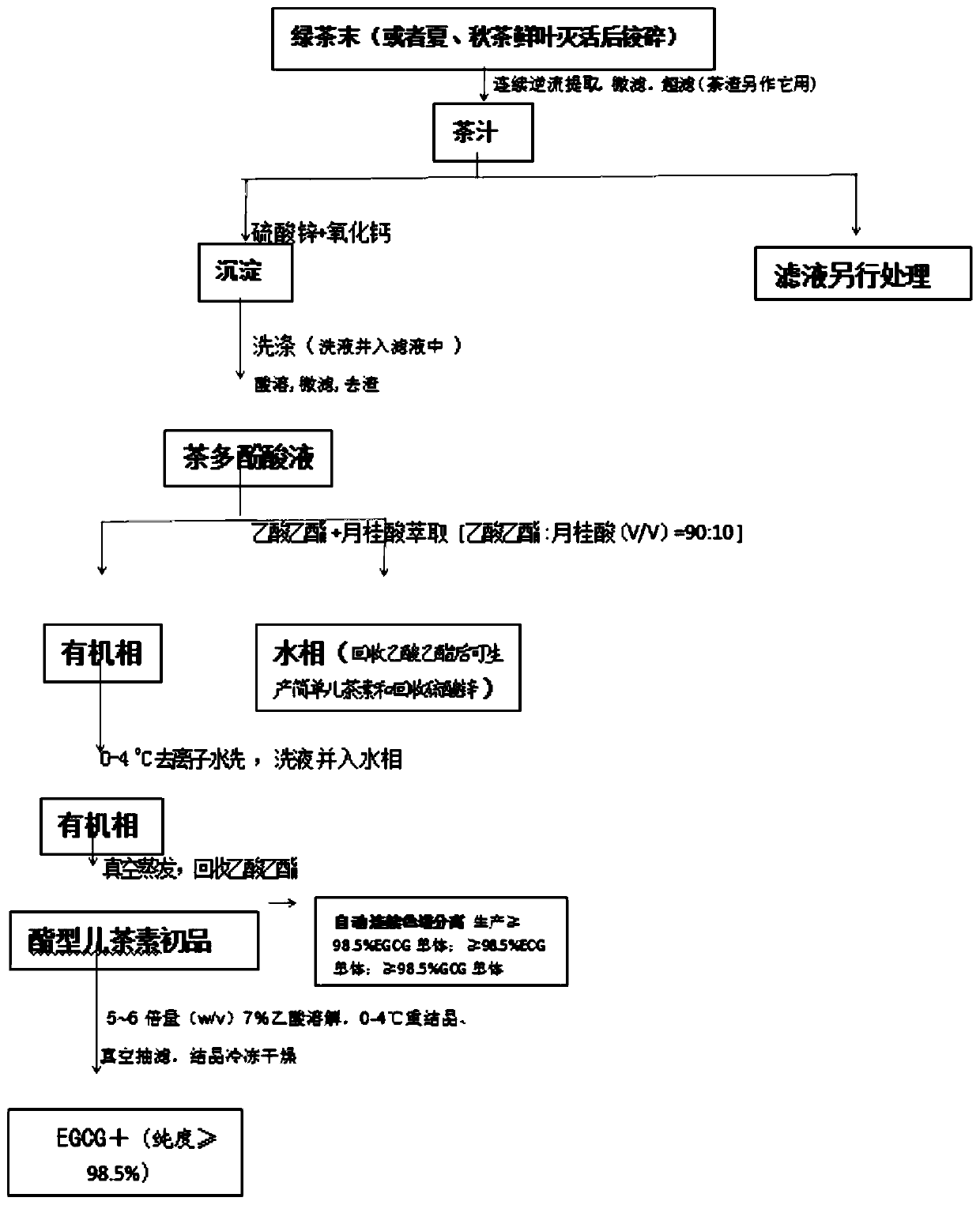
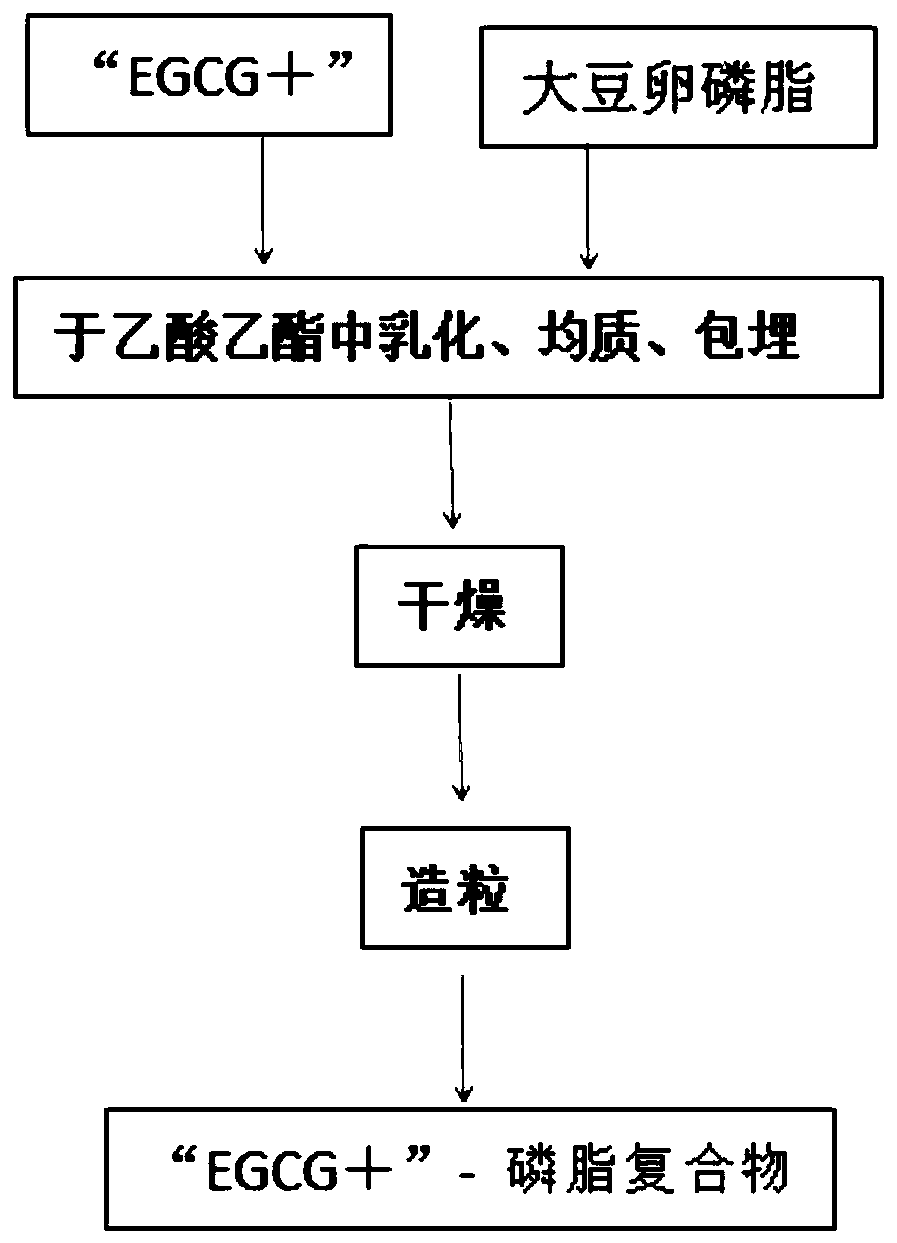
[0063] f. Preparation of "EGCG+":
[0064] ⑴Add 3.5 times of deionized hot water (60-70°C) to dissolve the obtained crude ester catechin (with stirring), cool down to room temperature, and filter out the precipitated needle-shaped white crystals (lauric acid) (lauric acid Return to e. tea polyphenolic acid solution to prepare crude ester catechins), continue to cool down to 1-4°C, let stand for 24 hours, crystallize, and vacuum filter to obtain solid crystals;
[0065] (2) Dissolve solid crystals with 5-6 times (w / v) 7% acetic acid at room temperature, recrystallize at 0-4°C, and vacuum filter to obtain crystals;
[0066] (3) Recrystallize once more according to (2), vacuum filter to obtain crystals, and freeze-dry the crystals to obtain white crystals "EGCG+". After HPLC detection, the catechin content is ≧98.5%, including 50.5-59% EGCG (epigallocatechin gallate), plus 22.5-26% ECG (epicatechin gallate), plus 10 -13% of GCG (gallocatechin gallate), plus 6.2-9.5% of EGC (epi...
Embodiment 1
[0099] The preparation example of embodiment 1 " EGCG+ "
[0100] ①Extraction of tea powder: Inactivate 800 kg of fresh summer tea leaves (one bud and three leaves) (equivalent to 200 kg of dry tea leaves), crush them into 20-40 meshes, and add them to continuous countercurrent ultrasonic equipment for extraction. The extraction temperature is 50-60°C; the extraction time is 1 hour; the water consumption is 1200 kg.
[0101] ②Preparation of tea juice: Microfilter the extract (including tea liquid and tea dregs) obtained in ① with a 0.5 μm microporous precision filter, wash with water 0.5-1 times the weight of tea leaves at 40-60 °C; Dalton membrane pore ultrafilter is used for ultrafiltration to remove impurities such as pectin and protein to obtain clear tea juice.
[0102] ③Precipitation and separation of double salt: In the tea juice obtained in ②, add zinc sulfate and calcium chloride double salt with a ratio of 3.5:1 of 5-7% of its weight; adjust its pH to 0.5-0.7M sodiu...
Embodiment 2
[0106] Preparation example of embodiment 2 "EGCG+"
[0107] ①Extraction of tea powder: 800 kg of fresh autumn tea leaves (one bud and three leaves) (equivalent to 200 kg of dry tea leaves) are inactivated, crushed into 20-40 meshes, and added to continuous countercurrent ultrasonic equipment for extraction. The extraction temperature is 50-60°C; the extraction time is 1 hour; the water consumption is 1200 kg.
[0108] ②Preparation of tea juice: Filter the extract (including tea liquid and tea dregs) obtained in ① with a 0.5 μm microporous precision filter, wash with water 0.5-1 times the weight of tea leaves at 40-60°C; Dalton membrane pore ultrafilter is used for ultrafiltration to remove impurities such as pectin and protein to obtain clear tea juice.
[0109] ③Precipitation and separation of double salt: In the tea juice obtained in ②, add zinc sulfate and calcium chloride double salt with a ratio of 3.5:1 of 5-7% of its weight; adjust its pH to 0.5-0.7M sodium bicarbonate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| glycemic index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com