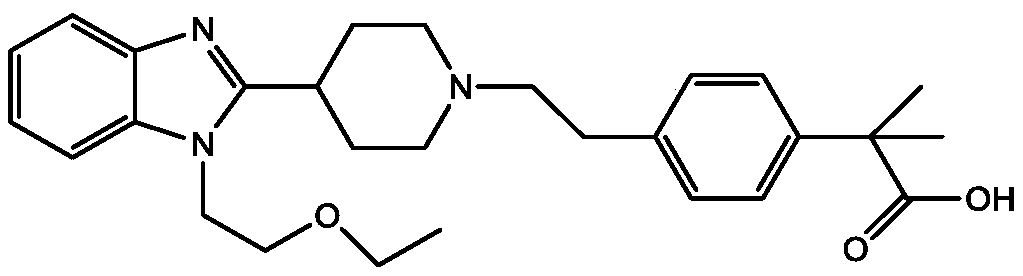
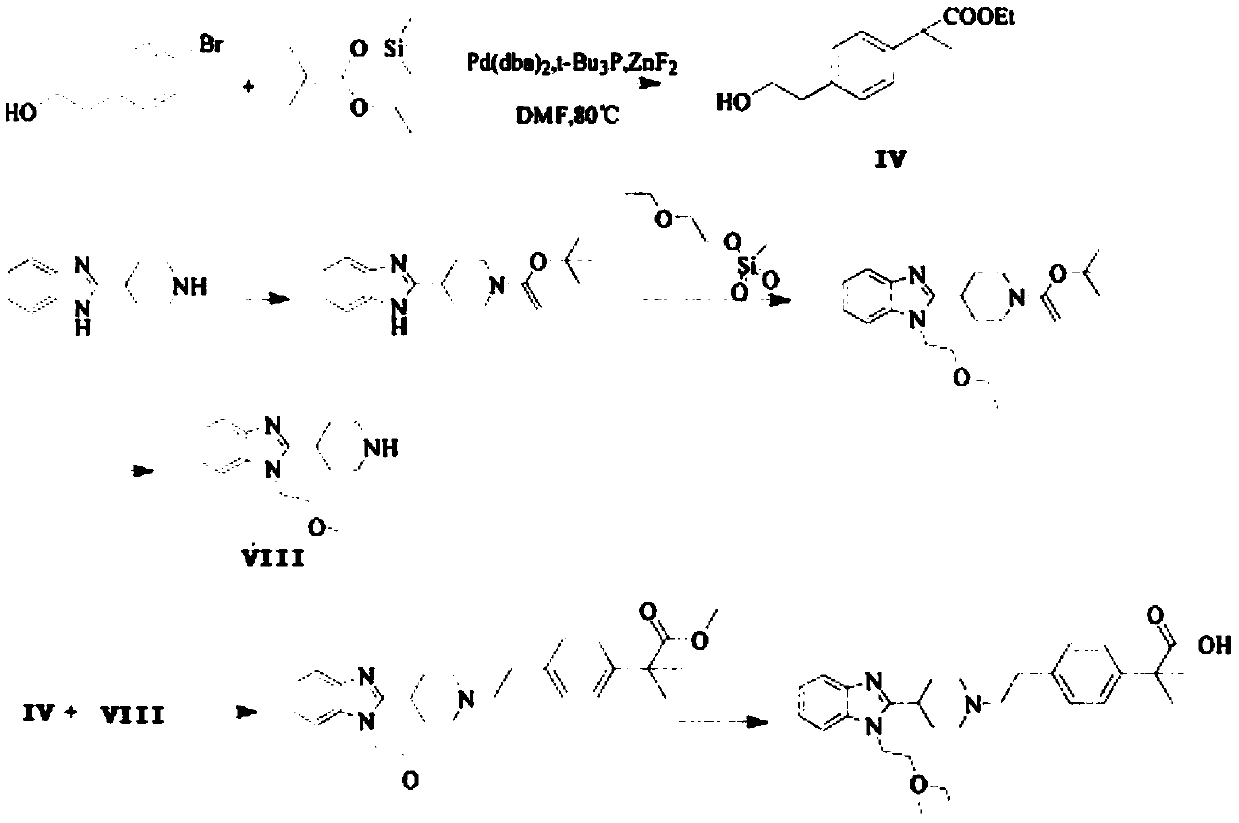
Preparation process of bilastine
A bilastine and preparation technology, applied in the field of medicine, can solve the problems of expensive raw materials, low purity and yield, and long reaction route, and achieve the effects of increasing yield, simplifying reaction steps, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
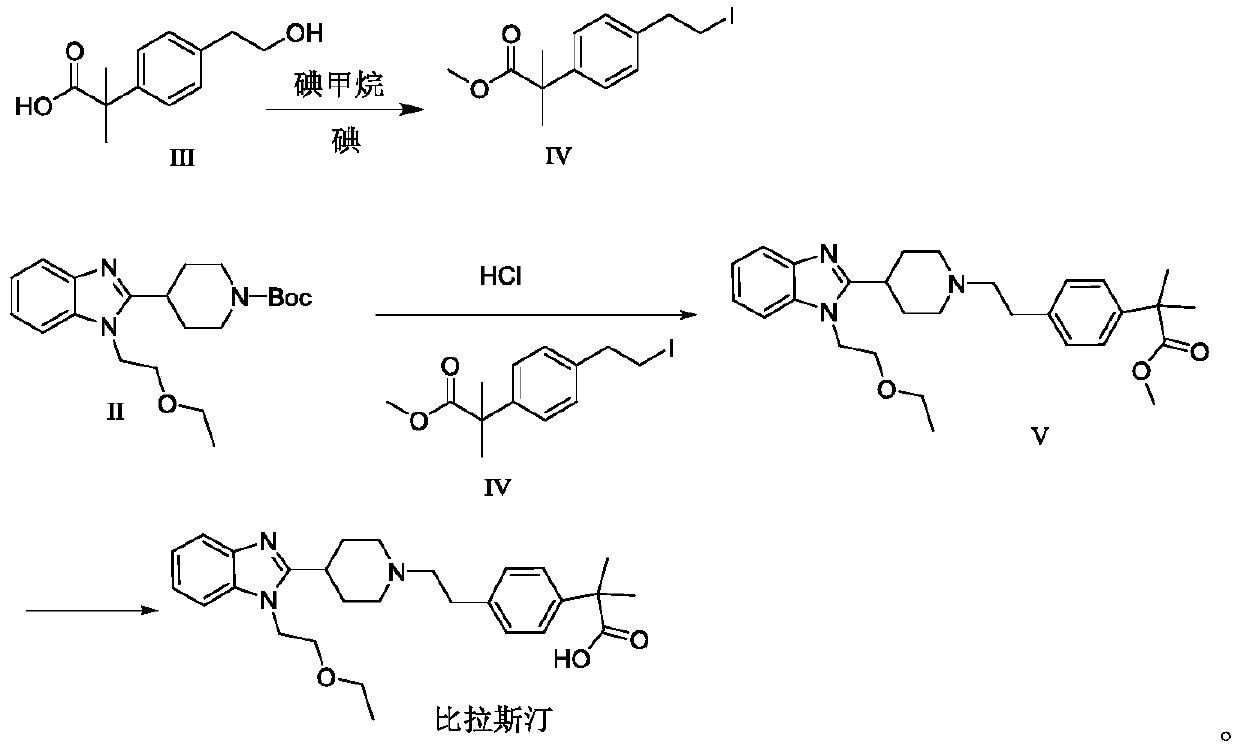
[0039] Embodiment 1: the preparation of compound VI
[0040] Add 10.41g of compound III and 52mL of DMF into a 250mL reaction flask, add 13.82g of potassium carbonate, add 7.10g of methyl iodide dropwise under stirring, stir at room temperature for 2h, TLC detects that the reaction is complete (petroleum ether: ethyl acetate=20:1, Rf= 0.35). Add 120 mL of water to the reaction solution, extract three times with 30 mL of ethyl acetate, combine the organic phases, dry with 8.0 g of anhydrous sodium sulfate, filter, concentrate the filtrate, add 80 mL of pre-dried dichloromethane, 19.67 g of triphenylphosphine and 1.70 g of After imidazole was stirred and dissolved, 19.04 g of iodine was added in 10 batches, stirred at room temperature for 2 h, TLC detected that the reaction was complete (petroleum ether: ethyl acetate = 20:1, Rf = 0.45), added 100 mL of saturated aqueous sodium bisulfite solution, and stirred for 30 min , liquid separation, the organic phase was washed successi...
Embodiment 2
[0041] Embodiment 2: the preparation of compound VI
[0042] Add 10.41g of compound III and 52mL of DMF into a 250mL reaction flask, add 8.10g of sodium methoxide, add 7.10g of methyl iodide dropwise under stirring, stir at room temperature for 2h, and TLC detects that the reaction is complete. Add 120 mL of water to the reaction solution, extract three times with 30 mL of ethyl acetate, combine the organic phases, dry with 8.0 g of anhydrous sodium sulfate, filter, concentrate the filtrate, add 80 mL of pre-dried dichloromethane, 20.98 g of triphenylphosphine and 3.40 g of After imidazole was stirred and dissolved, 19.04 g of iodine was added in 10 batches, stirred at room temperature for 2 h, TLC detected that the reaction was complete (petroleum ether: ethyl acetate = 20:1, Rf = 0.45), added 100 mL of saturated aqueous sodium bisulfite solution, and stirred for 30 min , liquid separation, the organic phase was washed successively with 100 mL of saturated aqueous sodium bisu...
Embodiment 3
[0043] The preparation of embodiment 3 compound IV
[0044]Add 10.41g of compound III and 52mL of DMF into a 250mL reaction flask, add 14.78g of lithium carbonate, add 7.10g of methyl iodide dropwise under stirring, stir at room temperature for 2h, and TLC detects that the reaction is complete. Add 120 mL of water to the reaction solution, extract three times with 30 mL of ethyl acetate, combine the organic phases, dry with 8.0 g of anhydrous sodium sulfate, filter, concentrate the filtrate, add 80 mL of pre-dried dichloromethane, 23.61 g of triphenylphosphine and 4.08 g of After imidazole was stirred and dissolved, 19.04 g of iodine was added in 10 batches, stirred at room temperature for 2 h, TLC detected that the reaction was complete (petroleum ether: ethyl acetate = 20:1, Rf = 0.45), added 100 mL of saturated aqueous sodium bisulfite solution, and stirred for 30 min , liquid separation, the organic phase was washed successively with 100 mL of saturated aqueous sodium bisu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com