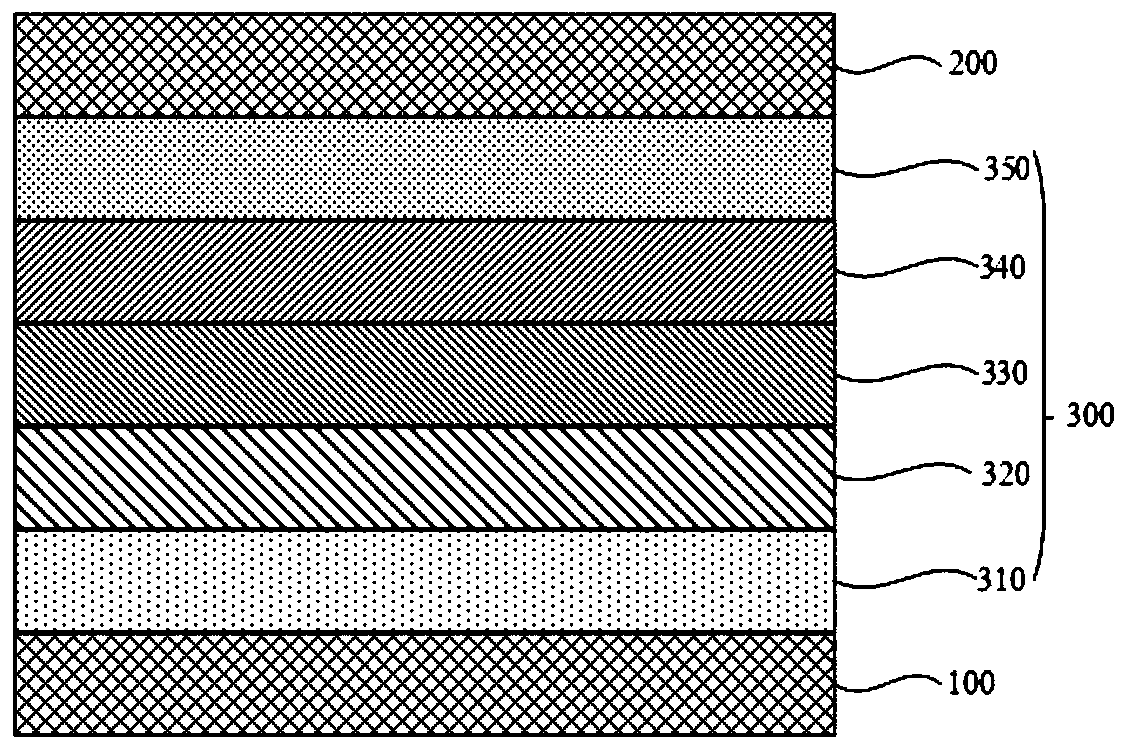
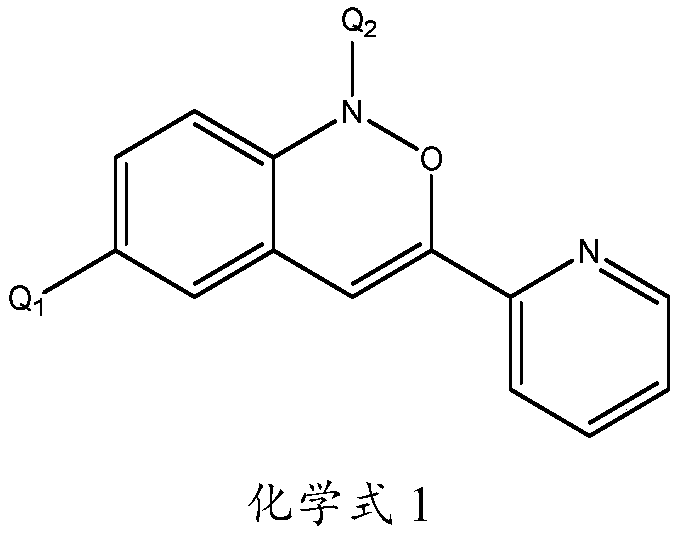
Benzoxazine compound, organic electroluminescent device, and electronic device
A benzoxazine and compound technology, which is applied in the field of organic electroluminescent materials to achieve the effects of good film formation, improved current efficiency, and difficulty in crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0070] The preparation example is used to illustrate the synthesis of starting material A.
[0071]
[0072]Add raw material 1 (56.4g, 0.206mol, CAS: 189748-25-2) and dichloromethane (500ml) into the reaction flask, heat up to 25°C under stirring, and add zinc powder (14.82g, 0.227mol), filter after reacting for 1h, add concentrated sulfuric acid (40.38g, 0.412mol) to the filtrate and stir for 30min, slowly add 1L of ice water to it, separate liquids, extract the aqueous phase with dichloromethane, wash the organic phase with water until neutral It was dried with anhydrous sodium sulfate, concentrated to dryness, and recrystallized with petroleum ether to obtain 42.9 g of solid material A with a purity of >98% and a yield of 69%. m / z=227.16[M+H] + ;Elemental analysis calculated value C 8 h 6 BrNO 2 , Theoretical C, 42.14; H, 2.65; Br, 35.04; N, 6.14; O, 14.03, Found C, 42.12; H, 2.64;
Embodiment 1
[0073] Embodiment 1: the synthesis of compound 2
[0074] (1)
[0075]
[0076] Under nitrogen atmosphere, raw material A (50g, 0.219mol) and tetrahydrofuran (500ml) were added to the reaction flask, and then water (100ml), potassium carbonate (60.61g, 0.438mol), raw material B were added in sequence 1 (43.37g, 0.219mol), tetrakis(triphenylphosphine)palladium (1.27g, 0.001mol), warming up to 80°C, stirring for 15h, the reaction was completed, adding water after cooling, extracting with dichloromethane, and extracting the extract with Dry over sodium sulfate for 1h, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize with ethanol to obtain 42.9g of Intermediate A 1 , purity > 98%, yield 65%.
[0077] (2)
[0078]
[0079] Intermediate A 1 (42.9g, 0.142mol) was added to the reaction flask under a nitrogen atmosphere, and then the raw material C was added in sequence 1 (33.18g, 0.142mol), DMF (429ml), 1,10-phenanthroline (0.28g, 0.0014...
Embodiment 2
[0086] Embodiment 2: the synthesis of compound 5
[0087] (1)
[0088]
[0089] Under nitrogen atmosphere, raw material A (50g, 0.219mol) and tetrahydrofuran (500ml) were added to the reaction flask, and then water (100ml), potassium carbonate (60.61g, 0.438mol), raw material B were added in sequence 2 (26.7g, 0.219mol), tetrakis(triphenylphosphine)palladium (1.27g, 0.001mol), warming up to 80°C, stirring for 13h, the reaction was completed, adding water after cooling, extracting with dichloromethane, extracting the extract with Dry over sodium sulfate for 1 hour, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize with ethanol to obtain 32.06g of Intermediate A 2 , purity > 98%, yield 65%.
[0090] (2)
[0091]
[0092] Intermediate A 2 (32.06g, 0.142mol) was added to the reaction flask under a nitrogen atmosphere, and then the raw materials C were added in sequence 2 (29.40g, 0.142mol), DMF (320.6ml), 1,10-phenanthroline (0.28g, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com