Biological synthesis method of branched ketose
A branched-chain ketose and microbial technology, applied in the field of branched-chain ketose biosynthesis, can solve the problems of high price of dihydroxyacetone, harsh reaction conditions, increased production costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
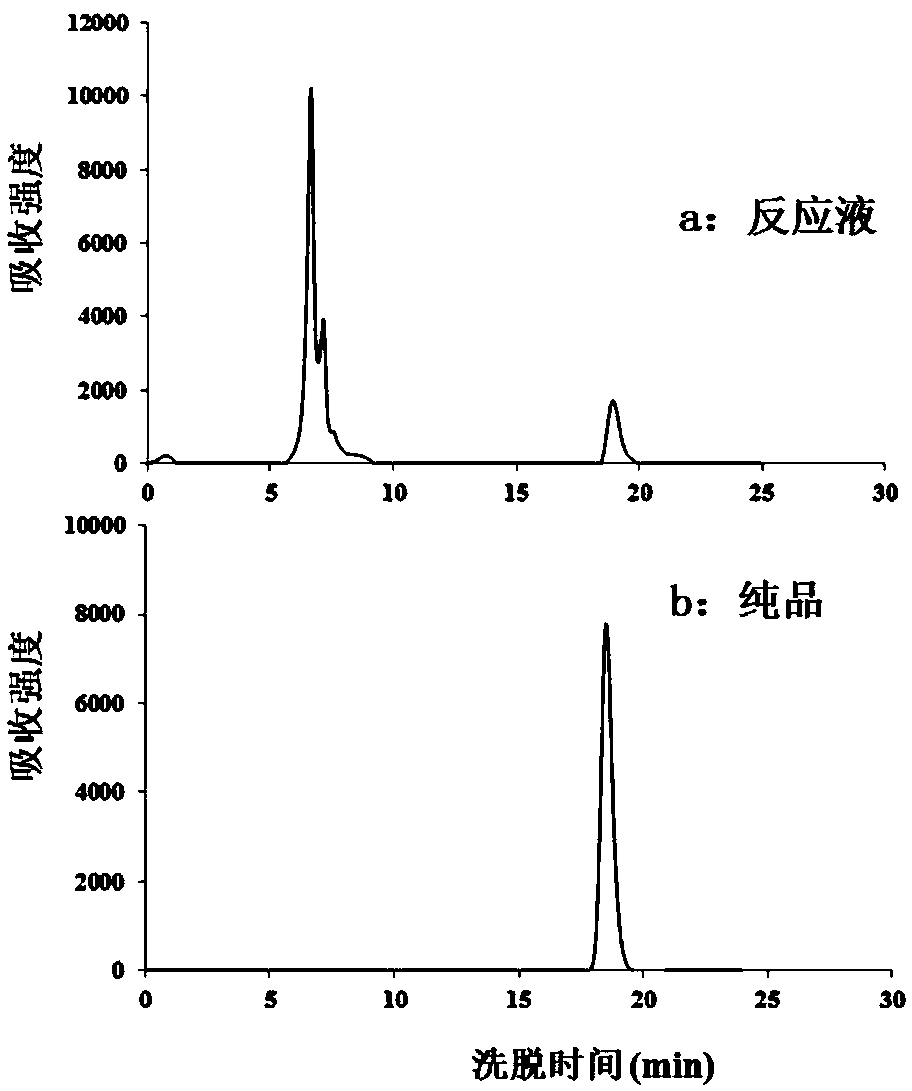
[0039] Embodiment 1, L-rhamnosan-1-phosphate aldolase characterization
[0040] 1. Construction of L-rhamnosan-1-phosphate aldolase recombinant strain
[0041] Primers were designed according to the L-rhamnosan-1-phosphate aldolase gene (SEQ ID NO: 5) derived from Escherichia coli MG1655 in Genbank to amplify the L-rhamnosan-1-phosphate derived from Escherichia coli 1-phosphate aldolase gene (rhaD) fragment, rhaD and pET21a were simultaneously digested with restriction enzymes NdeI and HindIII, and then ligated with T4 DNA ligase to obtain L-rhamnosugar-1-phosphate aldolase The carrier plasmid of the enzyme gene rhaD is named pET21-RhaD.
[0042] 2. The above-mentioned recombinant plasmid pET21-RhaD was transformed into Escherichia coli BL21(DE3) by chemical transformation, and two recombinant strains of Escherichia coli 21RhaD were obtained.
[0043] 3. Preparation of L-rhamnosan-1-phosphate aldolase
[0044] First, culture and induce Escherichia coli recombinant strain 21...
Embodiment 2
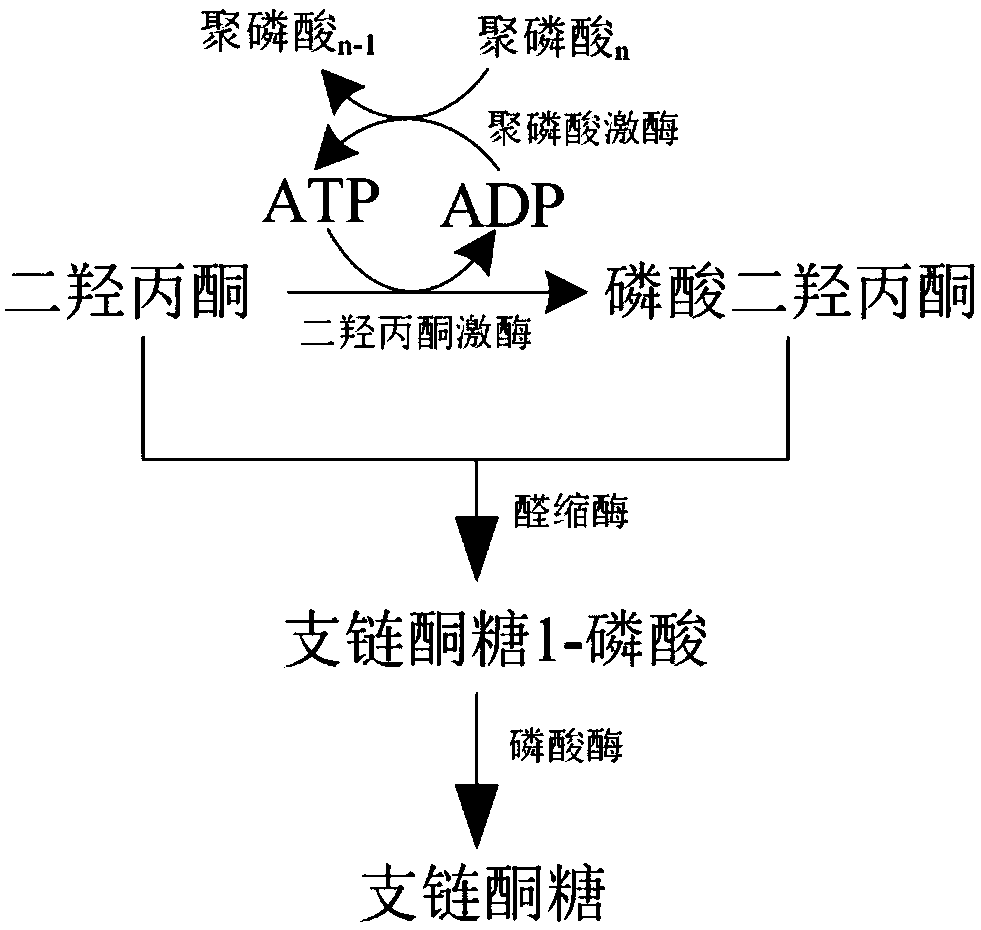
[0050] Example 2, multi-enzyme in vitro cascade catalyzes the synthesis of branched-chain ketose from dihydroxyacetone
[0051] Construct a multi-enzyme system composed of dihydroxyacetone kinase, L-rhamnose-1-phosphate aldolase, fructose 1-phosphatase and polyphosphate kinase to catalyze the synthesis of branched chain ketose from dihydroxyacetone, including The following steps:
[0052] 1. Construction of recombinant dihydroxyacetone kinase strain
[0053] Primers were designed according to the dihydroxyacetone kinase gene (SEQ ID NO: 10) derived from Citrobacter freundii in Genbank to amplify the dihydroxyacetone kinase gene (dhaK) fragment, and dhaK was simultaneously treated with restriction endonucleases NdeI and HindIII Digested with pET21a, and then ligated with T4 DNA ligase to obtain a vector plasmid containing dihydroxyacetone kinase gene dhaK, which was named pET21-DhaK.
[0054] 2. Construction of fructose 1-phosphatase recombinant strain
[0055] Primers were ...
Embodiment 3
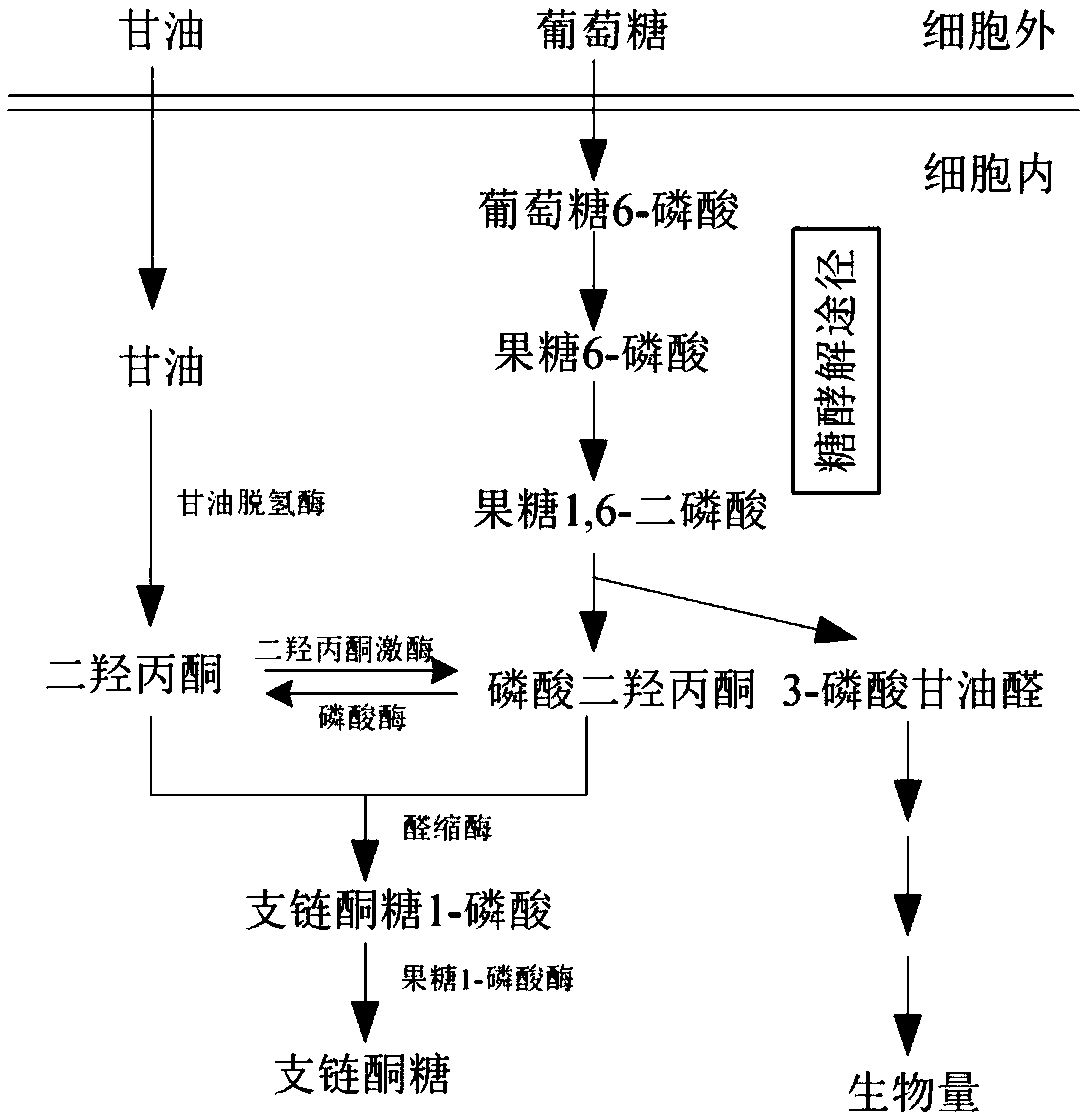
[0064] Embodiment 3, construction Corynebacterium glutamicum recombinant strain SY30
[0065] Construction of Recombinant Strain SY30 of Corynebacterium glutamicum
[0066] Corynebacterium glutamicum recombinant strain SY6 with triose phosphate isomerase gene knockout, its construction method is described in patent 201410055300.X, L-rhamnogum-1-phosphate aldolase gene was introduced into recombinant strain SY6, Phosphorylase gene and dihydroxyacetone phosphate phosphatase to obtain the recombinant strain of Corynebacterium glutamicum, named as bacterial strain S30, the construction scheme is as follows image 3 shown.
[0067] The specific construction process is as follows:
[0068] 1.1. According to the L-rhamnosan-1-phosphate aldolase gene (SEQ ID NO:5) and dephosphorylase (YqaB) gene (SEQ ID NO:6) derived from Escherichia coli MG1655 in Genbank ), derived from the sequence of dihydroxyacetone phosphate phosphatase (SEQ ID NO: 7) of Corynebacterium glutamicum, primers we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com