Charge repulsion induced single-chain collagen polypeptide probe and preparation method thereof
A technology of collagen polypeptide and chain collagen, which is applied to the preparation method of peptides, preparations for in vivo experiments, chemical instruments and methods, etc., can solve the damage of the tissue to be tested, the loss of collagen targeting binding ability of polypeptide probes, and the impact The accuracy of the test results and other issues can be avoided to avoid the risk of damage, the preparation is simple, and the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
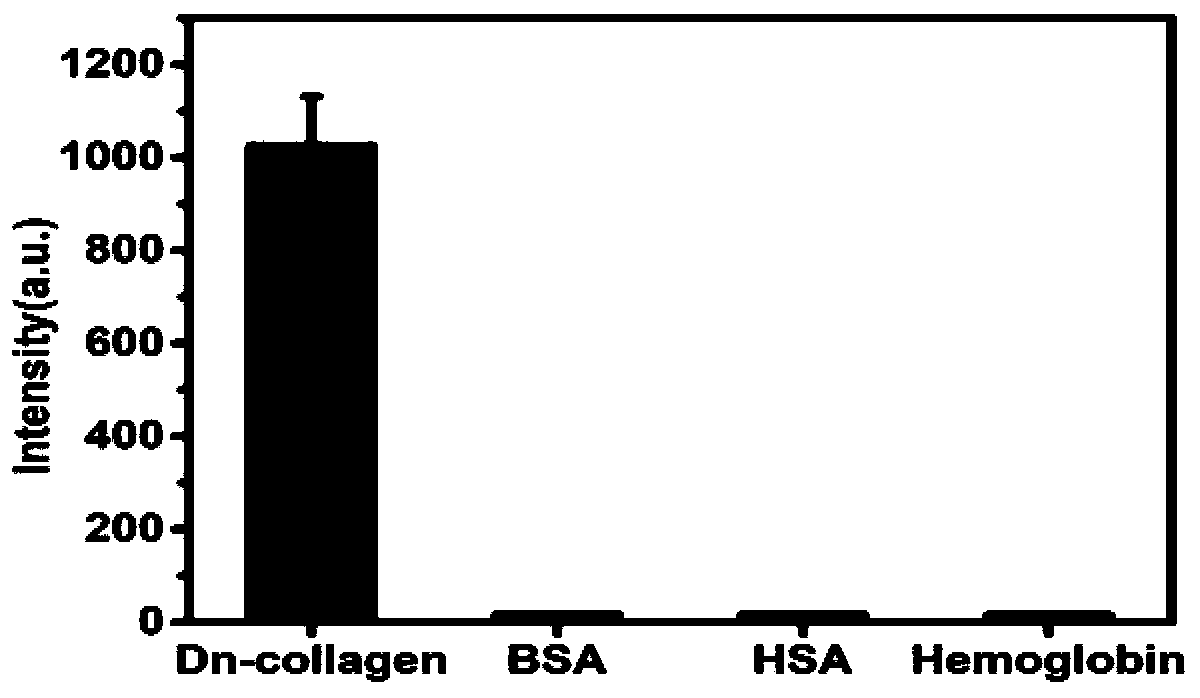
Examples
Embodiment 1
[0053] Example 1 Preparation of Collagen Polypeptide Probe F-PCTP-D1-6
[0054] 1. Sequence Design of Collagen Peptide Probe
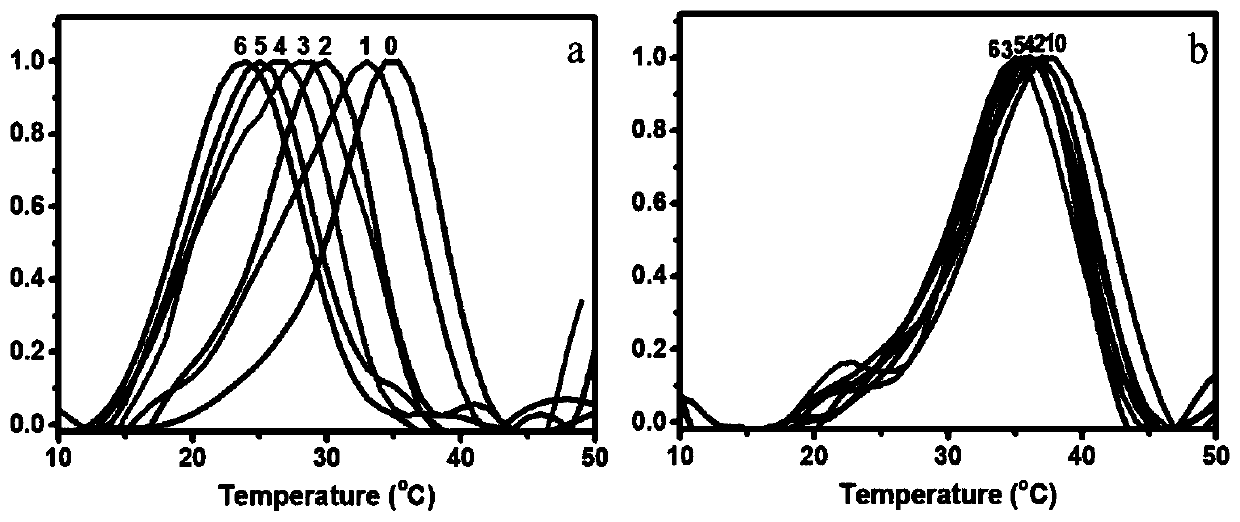
[0055] The peptide sequence of the collagen peptide probe is respectively designed as: FAM-Ahx-(GPO) 7 -(Asp) 6 -NH 2 、FAM-Ahx-(GPO) 7 -(Asp) 5 -NH 2 、FAM-Ahx-(GPO) 7 -(Asp) 4 -NH 2 、FAM-Ahx-(GPO) 7 -(Asp) 3 -NH 2 、FAM-Ahx-(GPO) 7 -(Asp) 2 -NH 2 、FAM-Ahx-(GPO) 7 -(Asp) 1 -NH 2 .
[0056] 2. Preparation of Collagen Peptide Probes
[0057] (1) Add 100 mg of Rink ammonia resin to a reactor with a sieve plate, and use 5 mL of dichloromethane to swell the resin;
[0058] (2) Remove the N-terminal Fmoc protecting group from 20% piperidine / N,N-dimethylformamide (DMF) solution, and detect the complete removal of the protecting group by color reaction;
[0059] (3) Dissolve the amino acid (4eq), HOBt (4eq) and HBTU (4eq) whose N-terminus is protected by Fmoc in DMF, activate at low temperature for 20 minutes, add DIEA (6eq) dropwise to the s...
Embodiment 2
[0074] Example 2 Preparation of Collagen Polypeptide Probe R-PCTP-D6
[0075] 1. Design of Collagen Peptide Probes
[0076] The designed collagen peptide sequence is 6-ROX-Ahx-(GPO) 7 -(Asp) 6 -NH 2 , wherein 6-ROX is 6-carboxy-X rhodamine; 2. Preparation of collagen polypeptide probe
[0077] (1) Add 100 mg of Rink ammonia resin into a reactor with a sieve plate, and use 5 mL of dichloromethane to swell the resin;
[0078] (2) Remove the N-terminal Fmoc protecting group from 20% piperidine / N,N-dimethylformamide (DMF) solution, and detect the complete removal of the protecting group by color reaction;
[0079] (3) Dissolve the amino acid (4eq), HOBt (4eq) and HBTU (4eq) whose N-terminus is protected by Fmoc in DMF, activate at low temperature for 20 minutes, add DIEA (6eq) dropwise to the solution, mix the solution and add it to the reactor Medium, reaction 3hrs;
[0080] (4) After the reaction finishes, the reaction solution is extracted from the reactor, and the resin ...
Embodiment 3
[0093] Example 3 Collagen polypeptide probe F-PCTP-(GDD) 3 preparation of
[0094] 1. Design of Collagen Peptide Probes
[0095]The designed collagen peptide sequence is FAM-Ahx-(GPO) 7 -(Gly-Asp-Asp) 3 -NH 2 , wherein FAM is carboxyfluorescein.
[0096] 2. Preparation of Collagen Peptide Probes
[0097] (1) Add 100 mg of Rink ammonia resin into a reactor with a sieve plate, and use 5 mL of dichloromethane to swell the resin;
[0098] (2) Remove the N-terminal Fmoc protecting group from 20% piperidine / N,N-dimethylformamide (DMF) solution, and detect the complete removal of the protecting group by color reaction;
[0099] (3) Dissolve the amino acid (4eq), HOBt (4eq) and HBTU (4eq) whose N-terminus is protected by Fmoc in DMF, activate at low temperature for 20 minutes, add DIEA (6eq) dropwise to the solution, mix the solution and add it to the reactor Medium, reaction 3hrs;
[0100] (4) After the reaction finishes, the reaction solution is extracted from the reactor, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com