Quantitative nuclear magnetic hydrogen spectrum valuing method of pentaerythritol standard substance
A pentaerythritol and standard substance technology, which is applied in the field of quantitative hydrogen nuclear magnetic spectrum determination of pentaerythritol purity standard substances, can solve the problems of difficult pentaerythritol signal, low determination accuracy, etc. Accurate and reliable setting value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
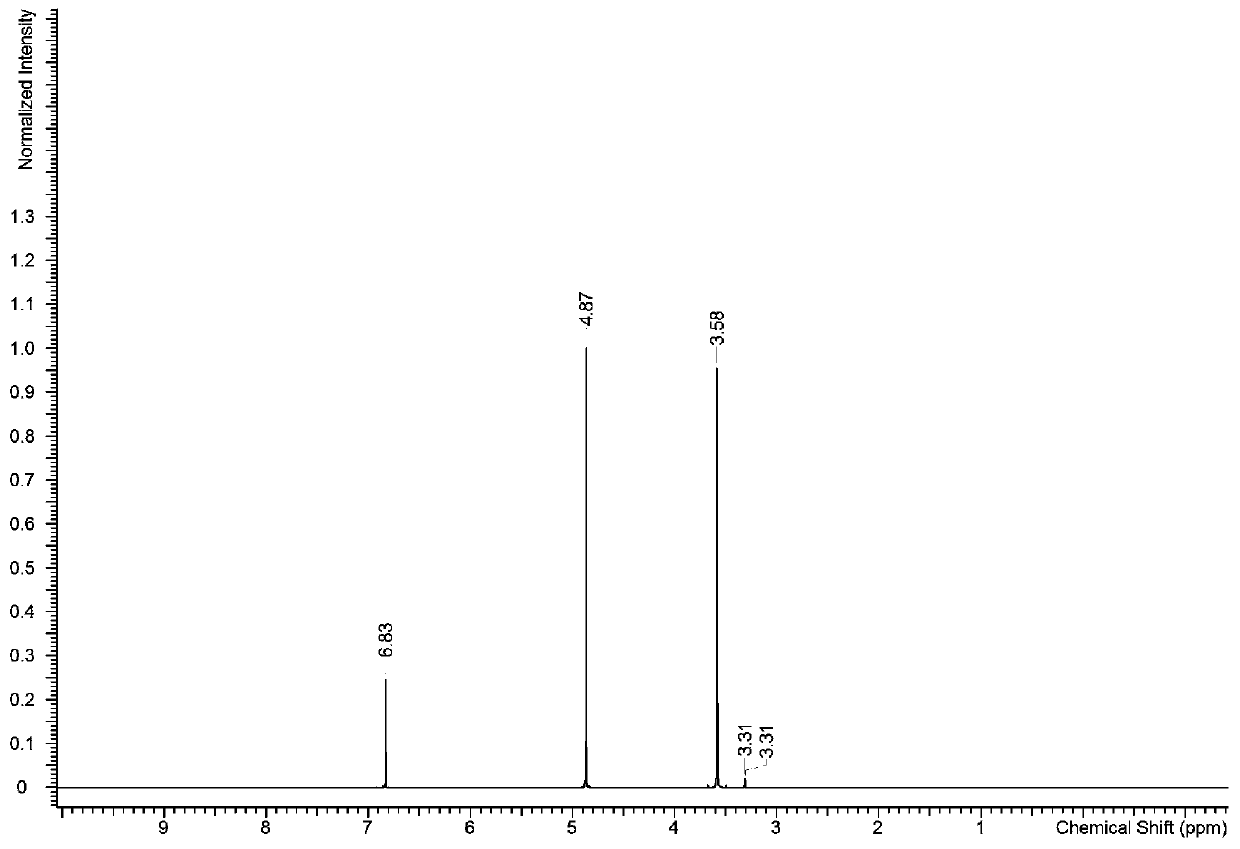
[0052] With hexamethyldisiloxane standard substance as internal standard substance, the purity of pentaerythritol standard substance was determined by quantitative NMR method.
[0053] 1.1 Instrument and sample preparation
[0054] ① Instrument: BrukerAscend 800 superconducting nuclear magnetic resonance spectrometer (German BRUKER company), MettlerToledo XP6 1 / 100,000 balance (Switzerland METTLER company);
[0055] ② 5mm standard NMR sample tube (NORELL, USA); hexamethyldisiloxane deuterated acetone solution standard (Xi’an Institute of Modern Chemistry, content 0.102%, U=2%), pentaerythritol standard (National Defense Science and Technology Industry Explosive One level metering station)
[0056] 1.2 Preparation and determination of NMR samples to be tested
[0057] Accurately weigh about 20 mg of pentaerythritol standard substance to be tested in a 5 mm NMR tube, then directly pipette about 0.6 ml of deuterated acetone standard solution of hexamethyldisiloxane and weigh, m...
Embodiment 2
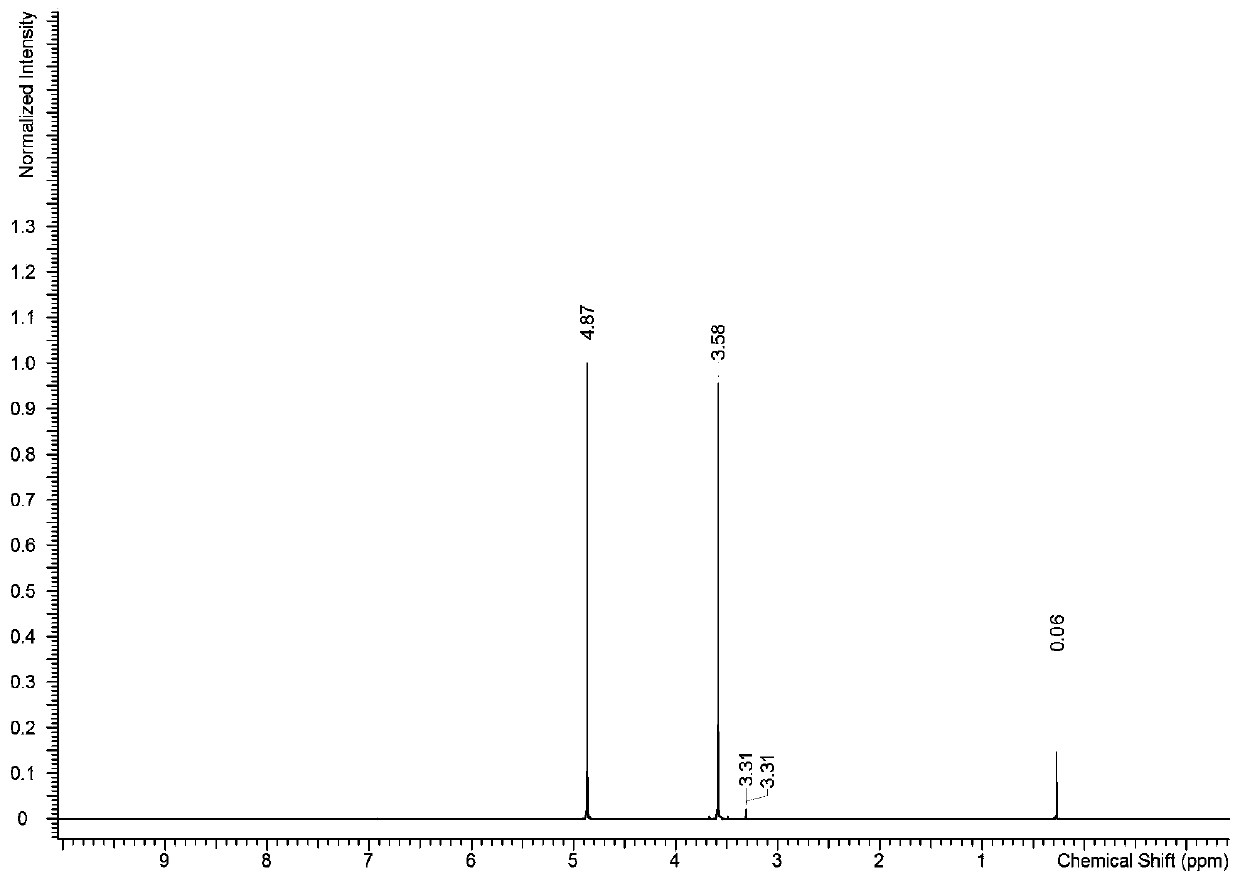
[0087] With fumaric acid purity standard substance as internal standard substance, the purity of pentaerythritol standard substance was determined by quantitative NMR method.
[0088] 2.1 Instrument and sample preparation
[0089] ① Instrument: BrukerAscend 800 superconducting nuclear magnetic resonance spectrometer (German BRUKER company), MettlerToledo XP6 1 / 100,000 balance (Switzerland METTLER company);
[0090] ② Deuterated methanol (deuterium degree > 99.8%, American CIL company); heavy water (deuteration degree > 99.8%, American CIL company) 5mm standard NMR sample tube (NORELL company in the United States); fumaric acid standard product (Germany Dr.Ehrensforfer, purity 99.9%), standard pentaerythritol (first-level metering station for explosives in national defense technology industry)
[0091] 1.2 Solution preparation
[0092] Accurately weigh about 150mg of the internal standard fumaric acid into a 10ml volumetric flask, then add a 2:1 mixed solvent of deuterated me...
Embodiment 3
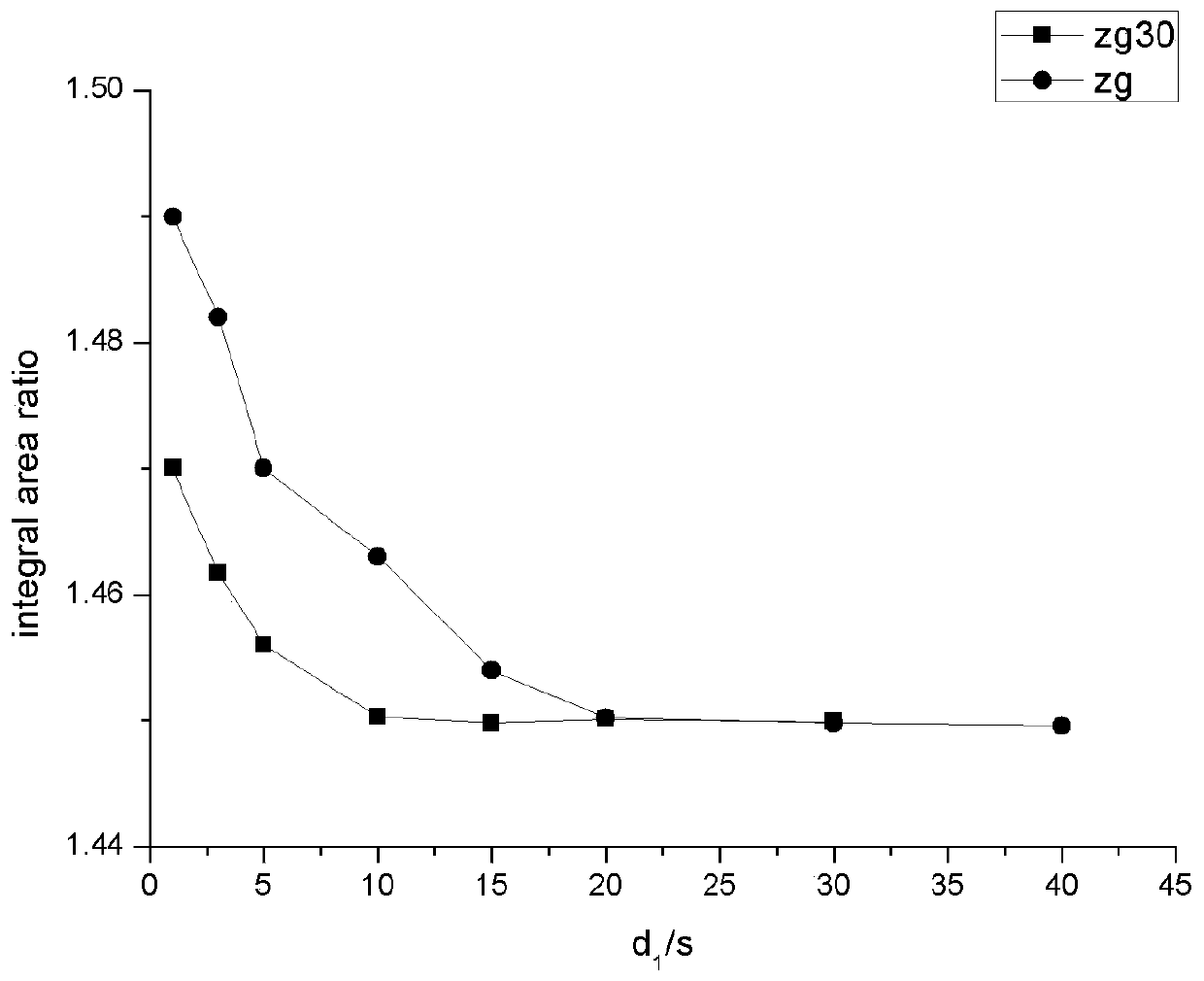
[0117] Since the relaxation of protons in different chemical environments in the NMR samples to be tested is different, the time required for the relaxation to fully return to equilibrium after the protons are excited in the magnetic field environment is different. In the classical NMR theory, it is required to use a 90-degree pulse to achieve complete relaxation in quantitative NMR experiments, and the pulse delay time (d 1 ) must be greater than five times the spin-lattice relaxation time (T 1 ). For the NMR samples to be measured of pentaerythritol as the internal standard of fumaric acid and hexamethyldisiloxane, an inversion recovery experiment (180 ° and 90 ° pulse, d 1 Range: 0.01s~20s) to T 1 Determination of the spin-lattice relaxation time T of the characteristic hydrogen atoms on several compounds 1 As shown in Table 4 below. Then if the data shown in Table 4 shows that no matter which internal standard is used, the pulse delay time (d 1 ) must reach at least 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com