Method for detecting 9-nitrominocycline in tigecycline for injection
A technology of nitrominocycline and tigecycline, which is applied in the detection field of 9-nitrominocycline in tigecycline for injection, and can solve the problem that 9-nitrominocycline cannot be quantified effectively And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
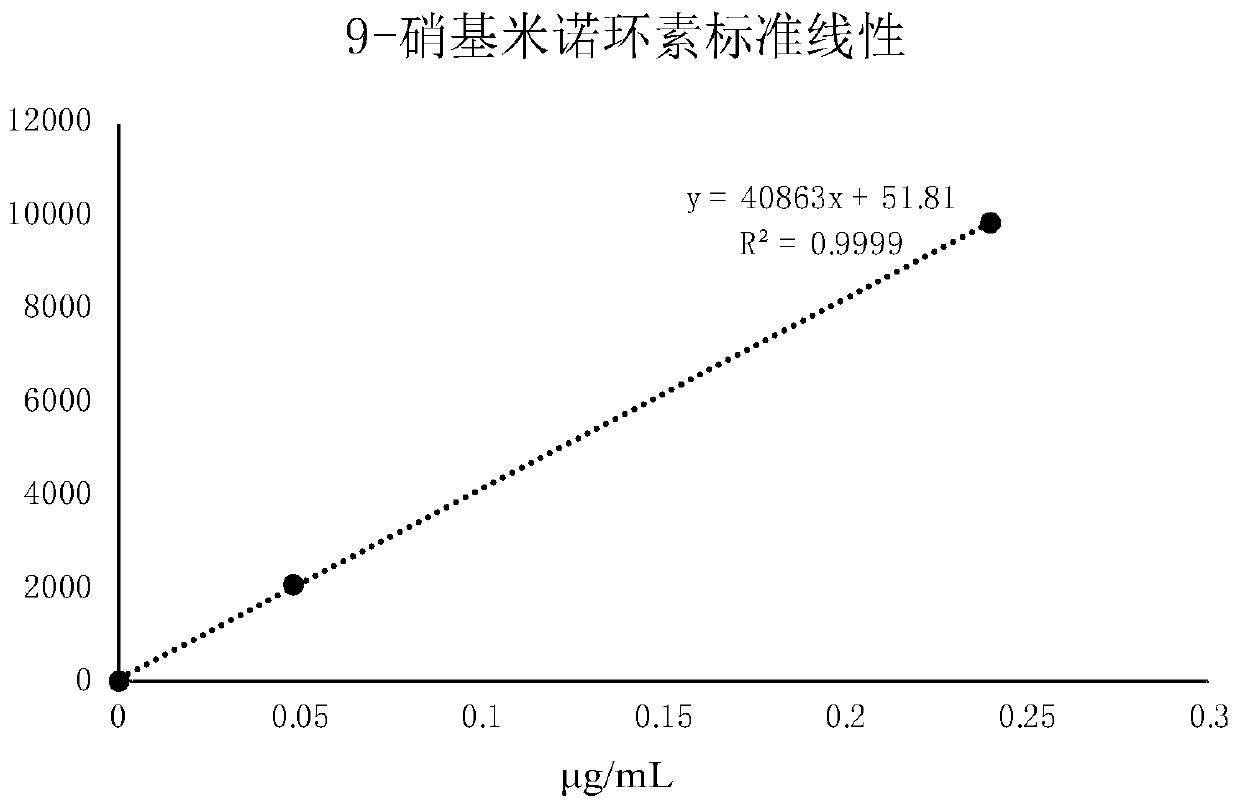
[0041] The process of establishing the standard curve
[0042] The standard solution preparation process is as follows:
[0043] The standard solution is the system suitability solution: use methanol to dilute 14.1 mg of 9-nitrominocycline standard sample (equivalent to 12 mg of 9-nitrominocycline) step by step, and prepare them respectively to a concentration of 0.05 μg / mL , a single standard solution of 0.25 μg / mL carries out the detection process of high performance liquid chromatography tandem mass spectrometry to the standard solution as follows:
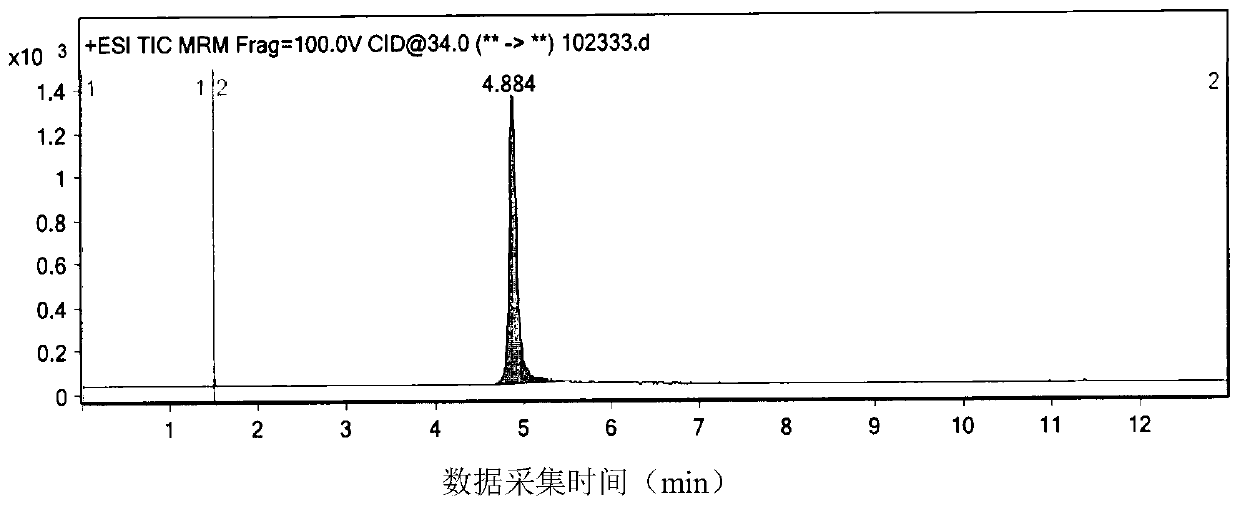
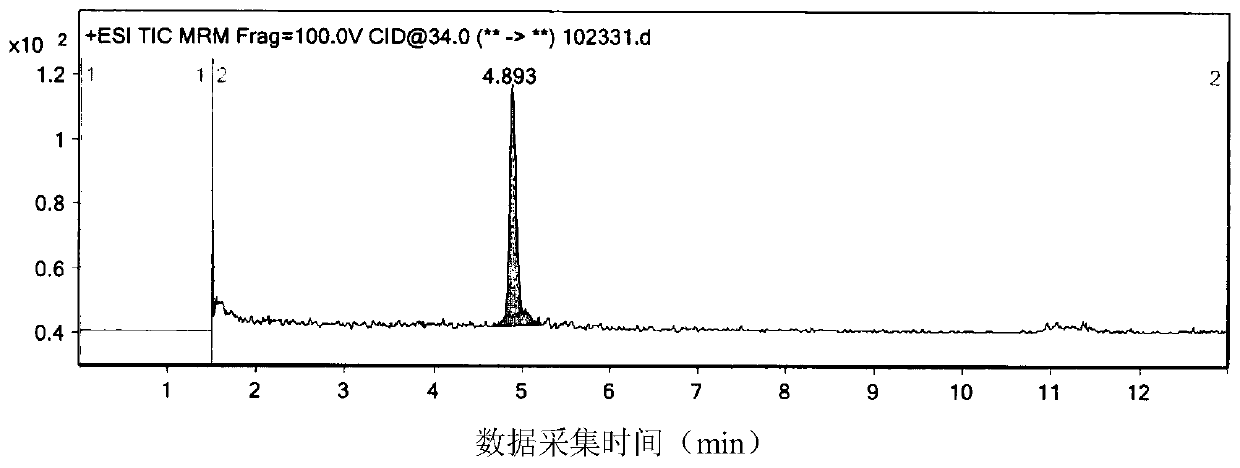
[0044] The conditions for HPLC detection are set as follows: the chromatographic column is a Welch XB-C18 chromatographic column, and the chromatographic column size is 4.6×50mm, 5μm, the column temperature is 35°C, the flow rate is 0.5ml / min, and the mobile phase A is volume The formic acid content is 0.1%, the mobile phase B is acetonitrile, and the gradient elution process is: 0 ~ 2min using 75% mobile phase A and 25% mob...
Embodiment 2
[0071] Different from the detection conditions of Example 1, the details are as follows.
[0072] The conditions for HPLC detection are set as follows: the chromatographic column is a Welch XB-C18 chromatographic column, and the chromatographic column size is 4.6×50mm, 5μm, the column temperature is 30°C, the flow rate is 0.3ml / min, and the mobile phase A is volume The formic acid content is 0.2%, the mobile phase B is acetonitrile, and the gradient elution process is: 0 ~ 2min using 70% mobile phase A and 30% mobile phase B for elution, 2 ~ 8min using 1% mobile phase A and 99% mobile phase B was used for elution, and 70% mobile phase A and 30% mobile phase B were used for elution in 8-13 minutes.
[0073] The conditions for mass spectrometry detection are set as follows: the ion source is AJS ESI, the drying gas temperature is 310°C, the drying gas flow rate is 9L / min, the atomizing gas pressure is 30psi, the sheath gas temperature is 330°C, and the sheath flow rate is 10L / mi...
Embodiment 3
[0076] Different from the detection conditions of Example 1, the details are as follows.
[0077] The conditions for HPLC detection are set as follows: the chromatographic column is a Welch XB-C18 chromatographic column, and the chromatographic column size is 4.6×50mm, 5μm, the column temperature is 40°C, the flow rate is 0.8ml / min, and the mobile phase A is volume The content is 0.05% formic acid, mobile phase B is acetonitrile, the gradient elution process is: 0 ~ 2min adopts 80% mobile phase A and 20% mobile phase B to elute, 2 ~ 8min adopts 3% mobile phase A and 97% mobile phase B was used for elution, and 80% mobile phase A and 20% mobile phase B were used for elution in 8-13 minutes.
[0078] The conditions for mass spectrometry detection are set as follows: the ion source is AJS ESI, the drying gas temperature is 280°C, the drying gas flow rate is 7L / min, the atomizing gas pressure is 40psi, the sheath flow gas temperature is 360°C, and the sheath flow flow rate is 12L / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com