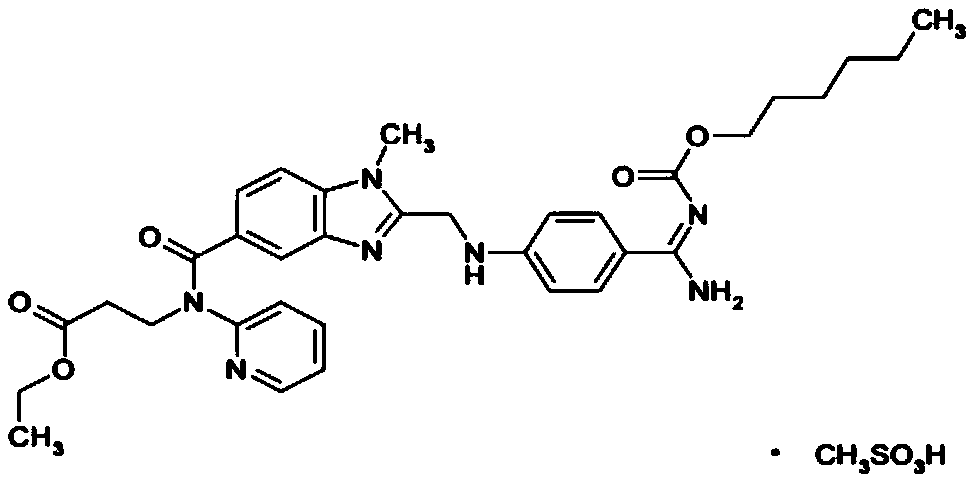
Dabigatran etexilate mesylate solid pharmaceutical preparation and preparation method thereof
A technology for dabigatran etexilate mesylate and solid pharmaceutical preparations, which is applied in the field of dabigatran etexilate mesylate solid pharmaceutical preparations and their preparation, can solve the problem that the requirements for parameter control are quite high, the non-compliance with green chemistry, and the production process Complex problems, etc., to achieve the effect of simple and controllable preparation method, saving tartaric acid auxiliary materials, and high production feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042]
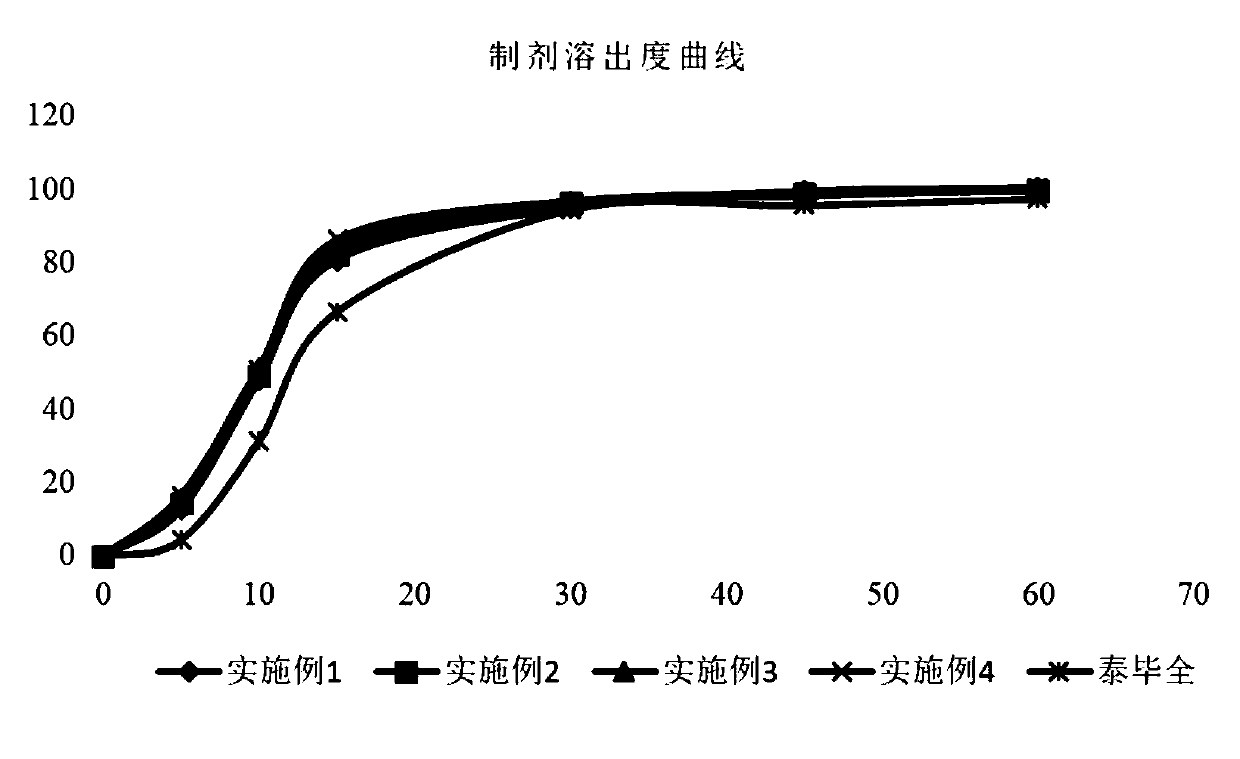
[0043] (1) The raw material drug dabigatran etexilate mesylate is micronized, the feed rate is set to be 10g / min, the feed pressure is 0.7-0.9MPa, and the crushing pressure is 0.6-0.8MPa; the final particle size of the control raw material The diameter is d50=3.17 μm, d90=4.83 μm.
[0044] (2) Weigh the prescribed amount of simethicone and add it to ethanol, then disperse and dissolve the prescribed amount of hydroxypropyl methylcellulose into the ethanol, and finally disperse the talcum powder into the above liquid, and keep stirring for 45 minutes to obtain the isolation layer coating liquid.
[0045] (3) Dissolve the prescribed amount of hydroxypropyl cellulose in isopropanol, then evenly disperse the prescribed amount of dabigatran etexilate into the above-mentioned liquid, finally add talcum powder therein, keep stirring for 45min, and 100-mesh sieve for subsequent use to obtain the drug-containing layer coating solution.
[0046] (4) Weigh the p...
Embodiment 2
[0050]
[0051] The weight of each component in embodiment 2 is according to the data in the table above, and the preparation steps are the same as in embodiment 1.
Embodiment 3
[0053]
[0054]
[0055] (1) The raw material drug dabigatran etexilate mesylate is micronized, the feed rate is set to be 10g / min, the feed pressure is 0.7-0.9MPa, and the crushing pressure is 0.6-0.8MPa; the final particle size of the control raw material The diameter is d50=4.86 μm, d90=9.27 μm.
[0056] All the other processing steps are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com