Ranitidine hydrochloride capsules and preparation method thereof
A technology of ranitidine hydrochloride and capsules, which is applied in the field of medicine, can solve problems such as damp heat instability, easy moisture absorption and accumulation, and achieve the effect of avoiding drug yellowing, improving stability and drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The embodiment of the present invention provides a kind of preparation method of ranitidine hydrochloride capsule, comprises the following steps:
[0057] S1. The materials in the prescribed amount are mixed in a fluidized state, and the materials at least include ranitidine hydrochloride;
[0058] S2. Keep the mixed material at a predetermined temperature, atomize and spray the wetting agent or binder solution, and granulate the material to form drug granules;
[0059] S3, drying the drug particles;
[0060] S4, making the dried drug granules into mixed granules; and
[0061] S5. Capsule filling of the mixed granules,
[0062] The steps of mixing, granulating and drying are carried out in the same airtight container.
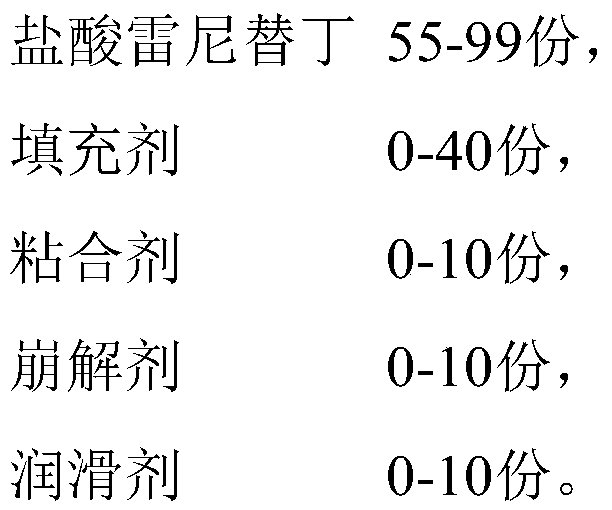
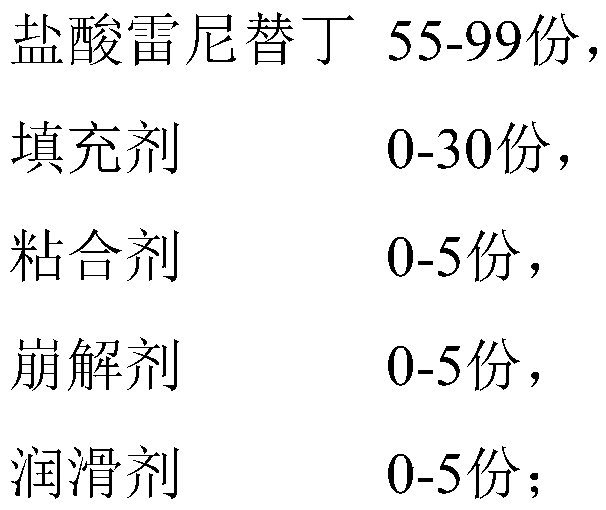
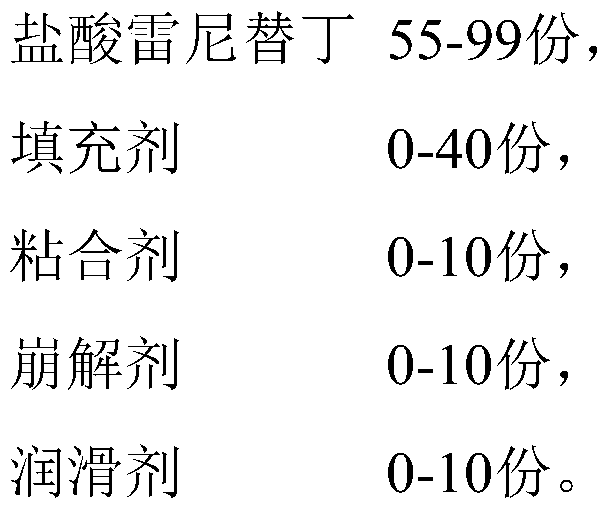
[0063]In the ranitidine hydrochloride capsules provided in the embodiments of the present invention and the preparation method thereof, the step S1 mixing, the step S2 granulation, and the step S3 drying are all completed in the same airtight containe...
Embodiment 1
[0091] prescription weight Ranitidine hydrochloride 99 copies Crospovidone 0.5 parts Magnesium stearate 0.5 parts Capsule content weight 169mg
[0092] Preparation:
[0093] S1, get the material ranitidine hydrochloride of recipe quantity in fluidized bed, make ranitidine hydrochloride form fluidized state;
[0094] S2. Heat the ranitidine hydrochloride to 35-45°C, atomize and spray water into the fluidized bed, the pressure of the atomized spray is 0.1MPa, and the spray speed of the atomized spray is 35ml / min, The atomized water is sprayed onto the ranitidine hydrochloride in a fluidized state, and the material is granulated so that the ranitidine hydrochloride forms drug granules;
[0095] S3. After the drug granules are in good condition, dry the ranitidine hydrochloride drug granules in a fluidized bed until the moisture content is not higher than 5%;
[0096] S4, take out the dried ranitidine hydrochloride drug granules from th...
Embodiment 2
[0099] prescription weight Ranitidine hydrochloride 83 copies starch 5 copies lactose 7 copies Calcium stearate 5 copies Capsule content weight 202mg
[0100] Preparation:
[0101] S1, get the material ranitidine hydrochloride and lactose of recipe quantity in fluidized bed, make the mixed material of ranitidine hydrochloride and lactose form fluidized state, mix 10 minutes;
[0102] S2. Heat the mixture of ranitidine hydrochloride and lactose to 25-35°C, atomize and spray starch slurry solution with a mass concentration of 5% into the fluidized bed, and spray the pressure of 0.3MPa, atomize The spray speed of injection is 60ml / min, and the material is granulated to form drug granules;
[0103] S3. After the drug granules are in good condition, dry the drug granules in a fluidized bed until the water content is not higher than 5%;
[0104] S4, the dried drug granules are taken out from the fluidized bed, crushed and granulated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com