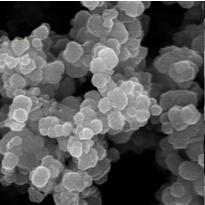
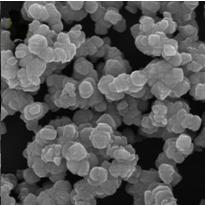
Method for preparing iron oxide red pigment and synchronously producing aromatic amine
A technology of red iron oxide and aromatic amine, applied in the field of fine chemicals, can solve the problems of large alkali consumption, polluted environment, poor reduction selectivity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 500mm of bottom water to a stirring reduction reactor with a diameter of 3000mm and a height of 3600mm and heat up to 80-85°C, 2800kg of solid FeSO 4 ·7H 2 Add water and heat up to about 70°C to dissolve O completely, and the volume is fixed to 3.76m³. Add 842Kg of DNS (4,4′-dinitrostilbene-2,2′-disulfonic acid) to 95°C to dissolve it completely and set the volume to 3.1m³. Add DNS and ferrous sulfate solution to the reactor at the same time and control the time at about 50 minutes, control the temperature at 85-87°C, and use lye to control the pH of the reaction system at 4-4.1. After adding the ferrous sulfate solution, add iron powder (80 mesh) for the first time, and after 10 minutes of reaction, control the time of adding the remaining DNS at 210 minutes, and control the temperature at 87-88°C, and the second and third times at 70 minutes and 140 minutes, respectively. Add the remaining iron powder three times. After adding DNS, keep it warm for 40 minutes an...
Embodiment 2
[0029] Add 500mm of bottom water to the reduction reactor with a diameter of 3000mm and a height of 3600mm with stirring and raise the temperature to 80-85°C to obtain 3.41m³ of ferrous chloride solution with a concentration of 2.8mol / L. DNS (4,4′-dinitrostilbene-2,2′-disulfonic acid) 842Kg, add water and raise the temperature to 95°C to dissolve it completely and set the volume to 3.1m³. DNS and ferrous chloride solution are added to the reactor at the same time, the time is controlled at about 50 minutes, the temperature is controlled at 85-87°C, and the pH is controlled at 4-4.1 by using lye. After adding the ferrous chloride solution, add iron powder (80 mesh) for the first time, and after 10 minutes of reaction, add the remaining DNS dropwise for 210 minutes, and control the temperature at 87-88°C, 70min and 140min for the second and third time Add the remaining iron powder three times. After adding DNS, keep it warm for 50 minutes and let it pour. Use plate and frame filt...
Embodiment 3
[0031] Add 500mm of bottom water to a stirring reduction reactor with a diameter of 3000mm and a height of 3600mm and raise the temperature to 80-85°C, FeSO 4 ·7H 2 Add 2800kg of water and raise the temperature to about 70°C to completely dissolve and set the volume to 3.76m³. DNS (4,4′-dinitrostilbene-2,2′-disulfonic acid) 842Kg is added to water and heated to 95°C, and the total dissolved volume is 3.1m³. DNS and ferrous sulfate solution are added to the reactor at the same time, the time is controlled at about 50 minutes, the temperature is controlled at 85-87 °C, and the pH is controlled at 4-4.1 with lye. After adding the ferrous sulfate solution, add iron powder (200 mesh) for the first time, and after 10 minutes of reaction, add the remaining DNS time control for 210 minutes, temperature control 87-88°C, 70min and 140min for the second and third time Add the remaining iron powder, after adding DNS, keep it warm for 30 minutes and let it pour. Plate and frame filtrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com