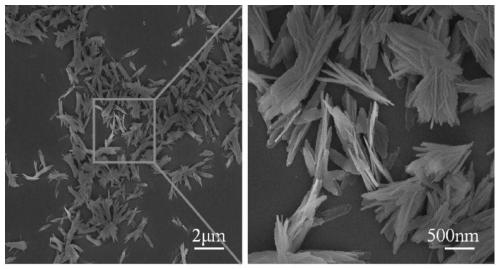
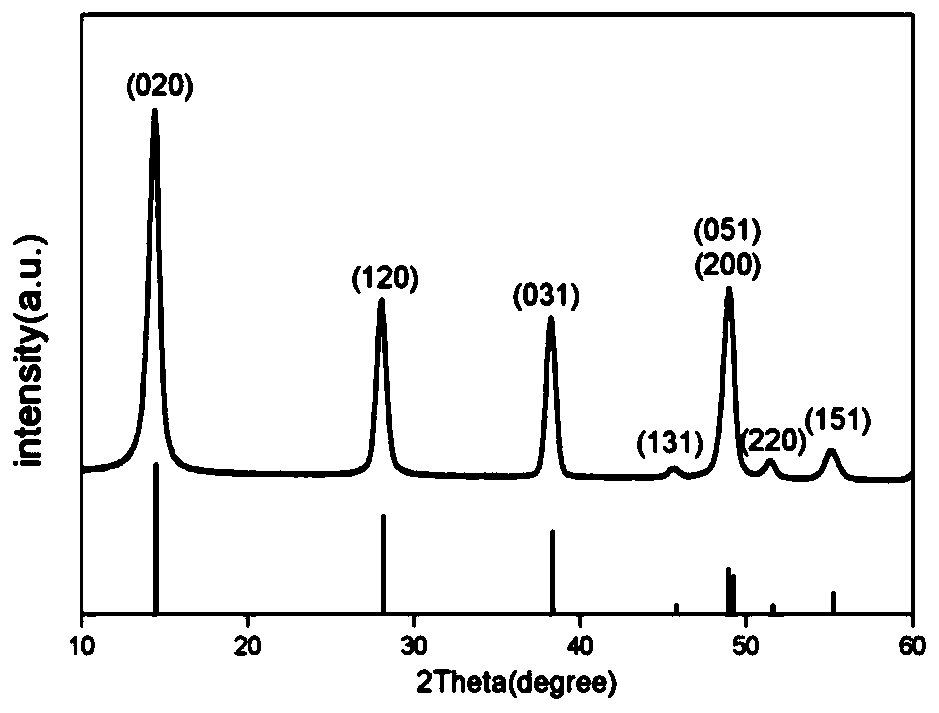
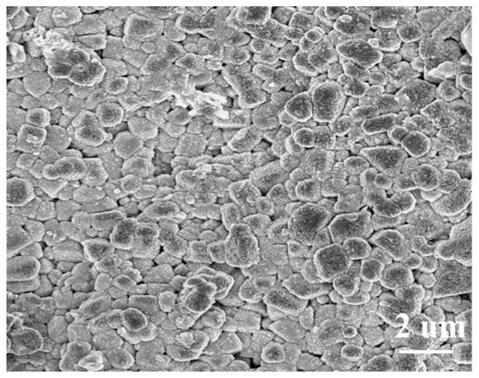
Lithium titanium aluminum phosphate solid electrolyte material and preparation method thereof
A solid electrolyte, lithium titanium aluminum phosphate technology, applied in solid electrolytes, non-aqueous electrolytes, circuits, etc., can solve the problems of low density lithium ion conductivity of solid electrolytes, high synthesis temperature, excessive energy consumption, etc., to achieve Improve the conductivity of lithium ions, lower the synthesis temperature, and prevent the loss of lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Aluminum nitrate and urea were used as raw materials and dissolved in deionized water according to the molar ratio of 1:1.5 stoichiometric ratio to obtain a mixed solution; then the mixed solution was placed in a reaction kettle and reacted at 200°C for 12 hours, and then The product is filtered, washed, and dried to obtain the AlOOH precursor; the AlOOH precursor obtained in step (1) with lithium carbonate, titanium dioxide and ammonium dihydrogen phosphate, according to Li, Al, Ti, P molar ratio is 1.3:0.3: 1.7:3 for mixing ingredients, then adding ethanol medium and ball milling, and sintering at 700°C for 6 hours in a dry air atmosphere to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 Powder, then the above Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 The bulk briquetting was formed, and the secondary sintering was carried out at 950 °C for 6 hours to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 solid electrolyte material.
Embodiment 2
[0039] Aluminum nitrate and urea were used as raw materials, and were dissolved in deionized water according to the molar ratio of 1:1.5 stoichiometric ratio to obtain a mixed solution; then the mixed solution was placed in a reaction kettle, and reacted at 180°C for 20 hours, and then The product is filtered, washed, and dried to obtain the AlOOH precursor; the AlOOH precursor obtained in step (1) with lithium carbonate, titanium dioxide and ammonium dihydrogen phosphate, according to Li, Al, Ti, P molar ratio is 1.3:0.3: 1.7:3 for mixing ingredients, then adding ethanol medium and ball milling, and sintering at 700°C for 6 hours in a dry air atmosphere to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 Powder, then the above Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 The bulk briquetting was formed, and the secondary sintering was carried out at 950 °C for 6 hours to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 solid electrolyte material.
Embodiment 3
[0041] Dissolve aluminum nitrate and urea in deionized water according to the stoichiometric molar ratio of 1:1.5 to obtain a mixed solution; then place the mixed solution in a reaction kettle and react at 220°C for 10 hours, and then The product is filtered, washed, and dried to obtain the AlOOH precursor; the AlOOH precursor obtained in step (1) with lithium carbonate, titanium dioxide and ammonium dihydrogen phosphate, according to Li, Al, Ti, P molar ratio is 1.3:0.3: 1.7:3 for mixing ingredients, then adding ethanol medium and ball milling, and sintering at 700°C for 6 hours in a dry air atmosphere to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 Powder, then the above Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 The bulk briquetting was formed, and the secondary sintering was carried out at 950 °C for 6 hours to obtain Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 solid electrolyte material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com