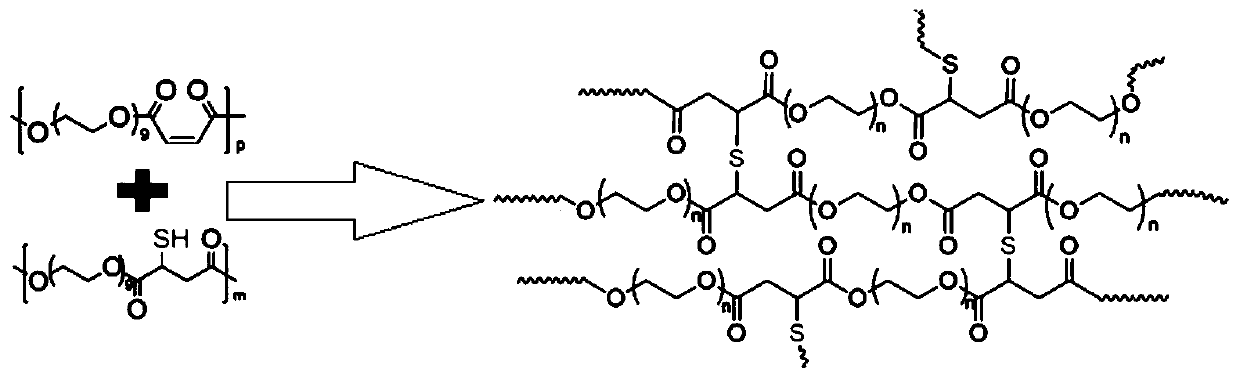
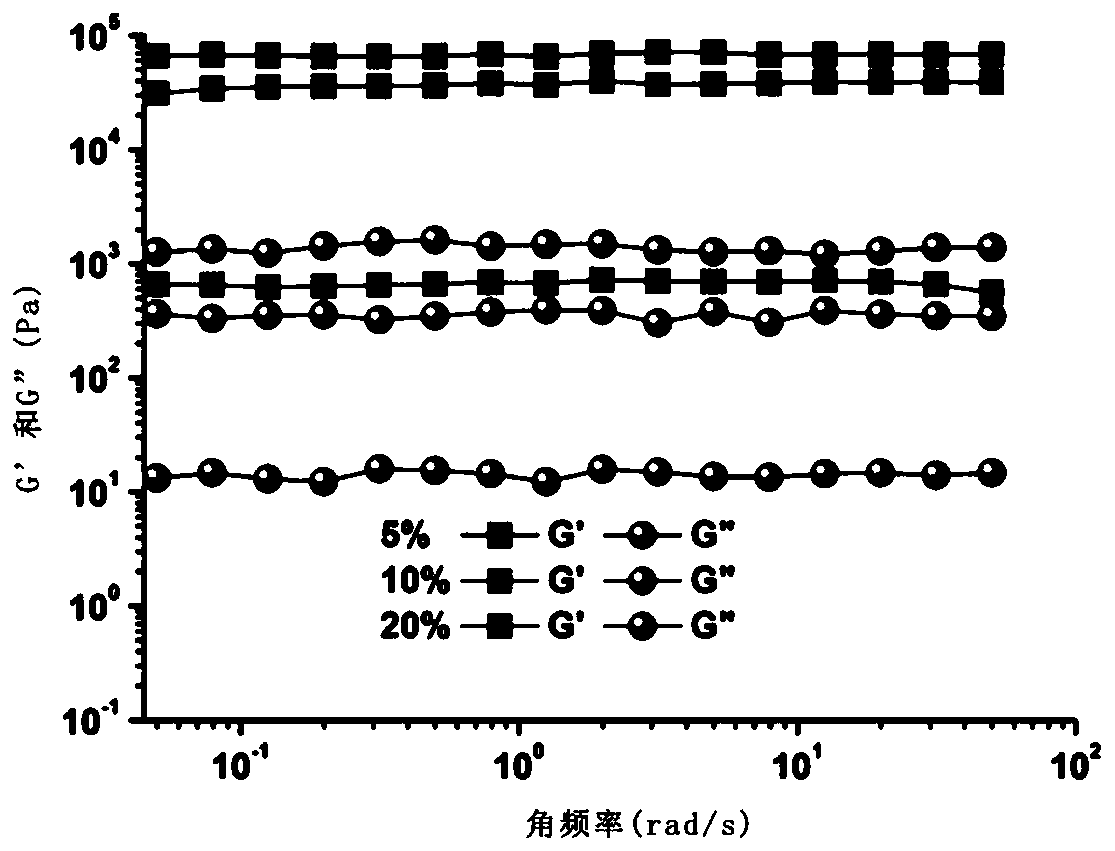
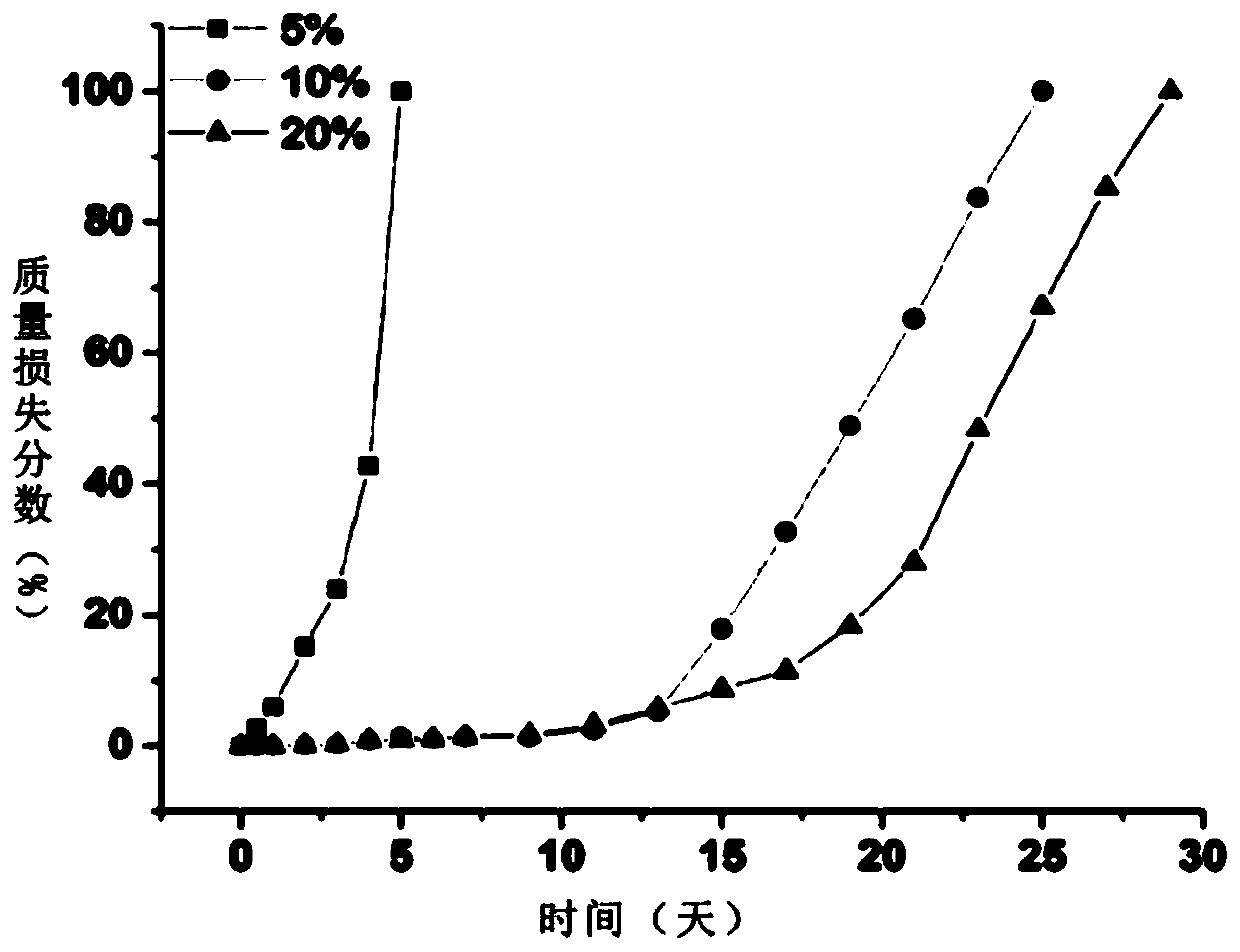
Preparation method of injectable polyethylene glycol active hydrogel
A technology of polyethylene glycol and oligoethylene glycol, which is applied in the field of medical material manufacturing, can solve the problems of high cost, complicated synthesis steps, and small number of functional groups, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and toluene in an oil bath at 110°C for azeotropic water removal, then add maleic acid (11.6g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours under the protection of nitrogen, then slowly raised the temperature to 100°C, and condensed and polymerized for 12 hours. The crude product was dissolved in water, placed in a cellulose acetate dialysis bag and dialyzed in 2L of 0.01M PBS for 1W to remove the catalyst. The interval frequency of water change is 1h (3 times), 2h (3 times), 6h (4 times), 12h (8 times), and 24h (2 times). Then freeze-dry in vacuum to obtain 41.6 g of the product POEGM. POEGM polycondensation reaction chemical formula:
[0018]
[0019] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and thiomalic acid (15.0g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours, then slowly raise...
Embodiment 2
[0023] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and toluene in an oil bath at 110°C for azeotropic water removal, then add maleic acid (11.6g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours under the protection of nitrogen, then slowly raised the temperature to 100°C, and condensed and polymerized for 12 hours. The crude product was dissolved in water, placed in a cellulose acetate dialysis bag and dialyzed in 2L of 0.01M PBS for 1W to remove the catalyst. The interval frequency of water change is 1h (3 times), 2h (3 times), 6h (4 times), 12h (8 times), and 24h (2 times). Then freeze-dry in vacuum to obtain 41.6 g of the product POEGM.
[0024] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and thiomalic acid (15.0g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours, then slowly raised the temperature to 120°C, condensed and polymerized for 12 ho...
Embodiment 3
[0027] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and toluene in an oil bath at 110°C for azeotropic water removal, then add maleic acid (11.6g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours under the protection of nitrogen, then slowly raised the temperature to 100°C, condensed and polymerized for 12 hours, dissolved the crude product in water, put it in a cellulose acetate dialysis bag and put The catalyst was removed by dialyzing for 1 W against 0.01 M PBS. The interval frequency of water change is 1h (3 times), 2h (3 times), 6h (4 times), 12h (8 times), and 24h (2 times). Then freeze-dry in vacuum to obtain 41.6 g of the product POEGM.
[0028] OEG in a 250mL eggplant bottle 9 (40.0g, 1.0mol) and thiomalic acid (15.0g, 1.0mol) and scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (5.0g, 1.0mol), esterified at 80°C for 12 hours, then slowly raised the temperature to 120°C, and condensed and polymerized fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com