Antibacterial material for pediatric pneumonia protection and manufacturing method thereof
The technology of an antibacterial material and a manufacturing method is applied in the field of antibacterial materials for paediatric pneumonia protection and its manufacturing, which can solve the problems of insufficient biosafety, fast release speed, short antibacterial time period, etc., achieve safe and long-term antibacterial effect, and ensure good health. , the effect of excellent antibacterial properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
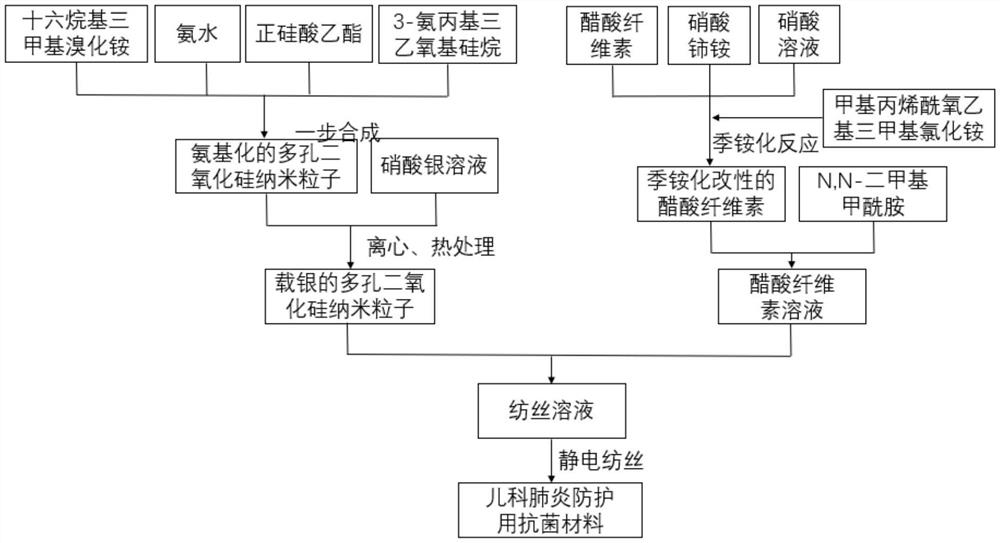
[0044] see figure 1 , the present embodiment provides a kind of manufacture method of antibacterial material for pediatric pneumonia protection, comprising the steps of:
[0045] S1. Dissolve 0.01mol of cetyltrimethylammonium bromide in 200mL of 50% ethanol solution, and then sequentially add 25% ammonia solution prepared by 0.0025mol of ammonia and deionized water into the solution , 0.02mol ethyl orthosilicate and 0.01mol 3-aminopropyltriethoxysilane, stirred at room temperature for 12 hours, then heated up to 80°C and stirred for 24 hours, after the solution was naturally cooled to room temperature, firstly at pH 6 Dialyzed in the acetic acid-ethanol solution for 24 hours to remove cetyltrimethylammonium bromide, and then dialyzed in deionized water for 24 hours, and freeze-dried the obtained solution at a vacuum degree of 5 Pa and a temperature of -20°C for 24 hours. Aminated porous silica nanoparticles are obtained;
[0046] S2. Disperse the aminated porous silica nanop...
Embodiment 2~5 and comparative example 1
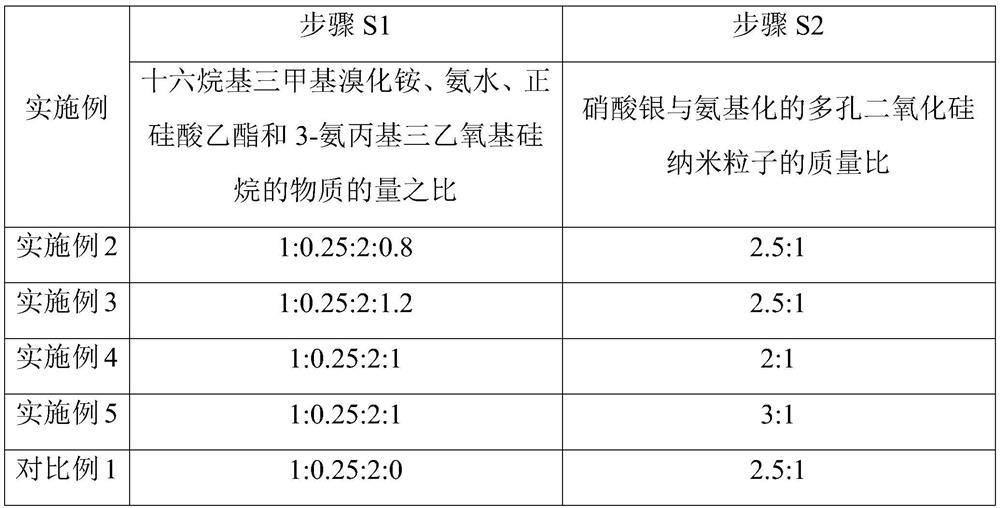
[0054] Embodiments 2-5 and Comparative Example 1 respectively provide a method for manufacturing an antibacterial material for pediatric pneumonia protection. Compared with Example 1, the difference is that Embodiments 2-5 only change the substances of each raw material in step S1 The ratio of the amount or the mass ratio of silver nitrate and aminated porous silica nanoparticles in step S2, comparative example 1 only changes the dosage of 3-aminopropyl triethoxysilane into 0 in step S1, and the rest The steps are all the same as those in Example 1, and will not be repeated here; the corresponding parameters corresponding to steps S1 and S2 in each example and comparative example are shown in Table 2.
[0055] Table 2 Parameters corresponding to Step S1 and Step S2 in Examples 2-5 and Comparative Example 1
[0056]
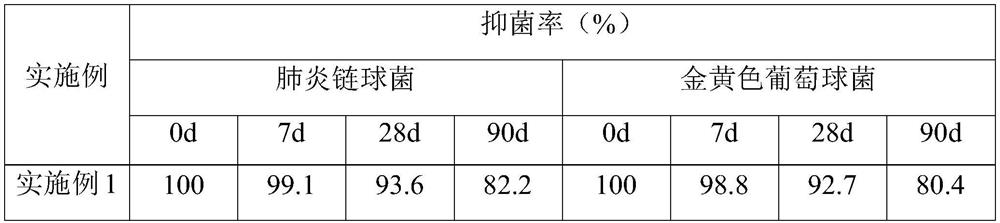
[0057] According to the same method as Example 1, the antibacterial properties of the antibacterial materials for pediatric pneumonia protection manufactured i...
Embodiment 6~9 and comparative example 2
[0063] Embodiments 6-9 and Comparative Example 2 respectively provide a method for the manufacture of antibacterial materials for pediatric pneumonia protection. Compared with Example 1, the difference is that Embodiments 6-9 only change the cellulose acetate and The mass ratio of methacryloyloxyethyltrimethylammonium chloride or the reaction temperature of quaternization reaction, comparative example 2 only changes the dosage of methacryloyloxyethyltrimethylammonium chloride in step S3 is 0, and the rest of the steps are consistent with that of Example 1, and will not be repeated here; the corresponding parameters corresponding to step S3 in each example and comparative example are shown in Table 4.
[0064] Table 4 Parameters corresponding to Step S3 in Examples 6-9 and Comparative Example 2
[0065]
[0066]
[0067] According to the same method as Example 1, the antibacterial properties of the antibacterial materials for pediatric pneumonia protection manufactured in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com