Synthesis method of rivaroxaban
A synthesis method and technology of rivaroxaban, applied in the production of bulk chemicals, organic chemistry and other directions, can solve the problems of unfavorable industrial production, difficult to obtain, high price, etc., to avoid the use of expensive raw materials, improve yield, improve The effect of production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
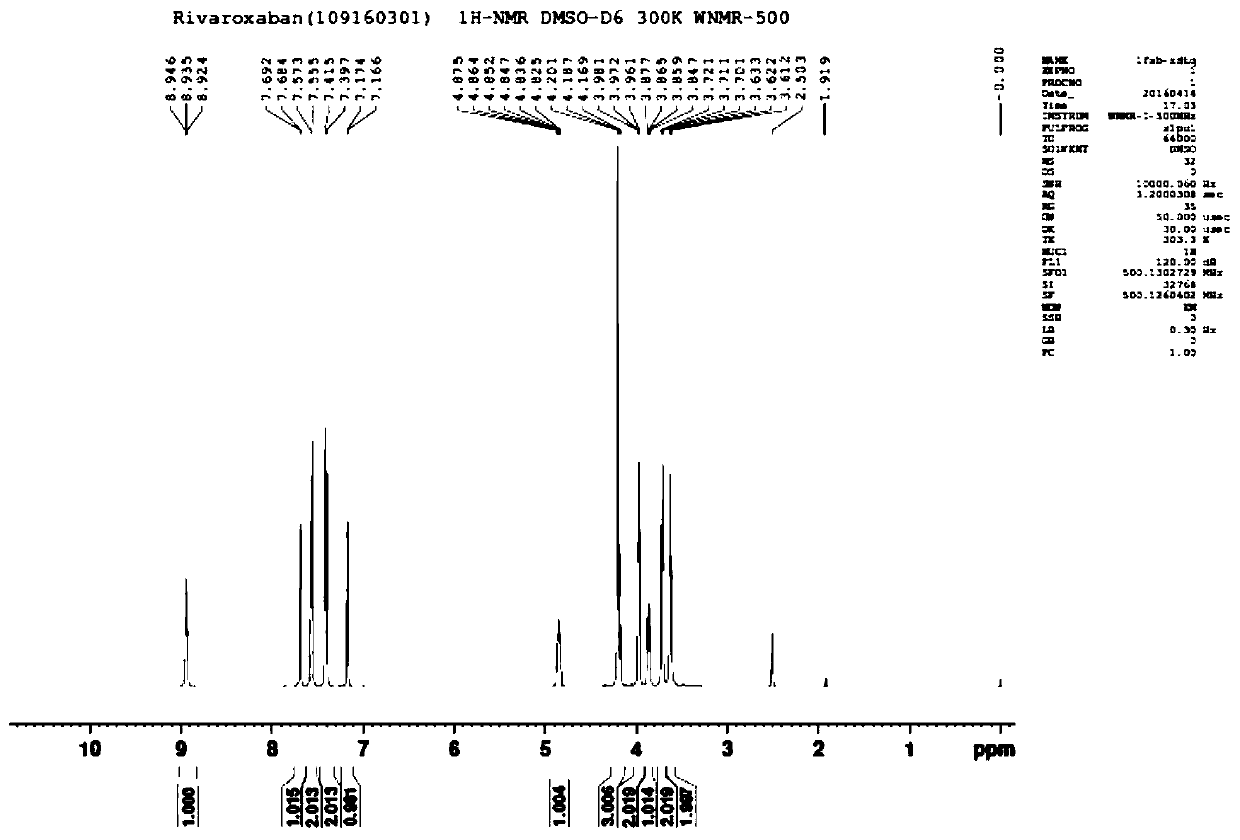
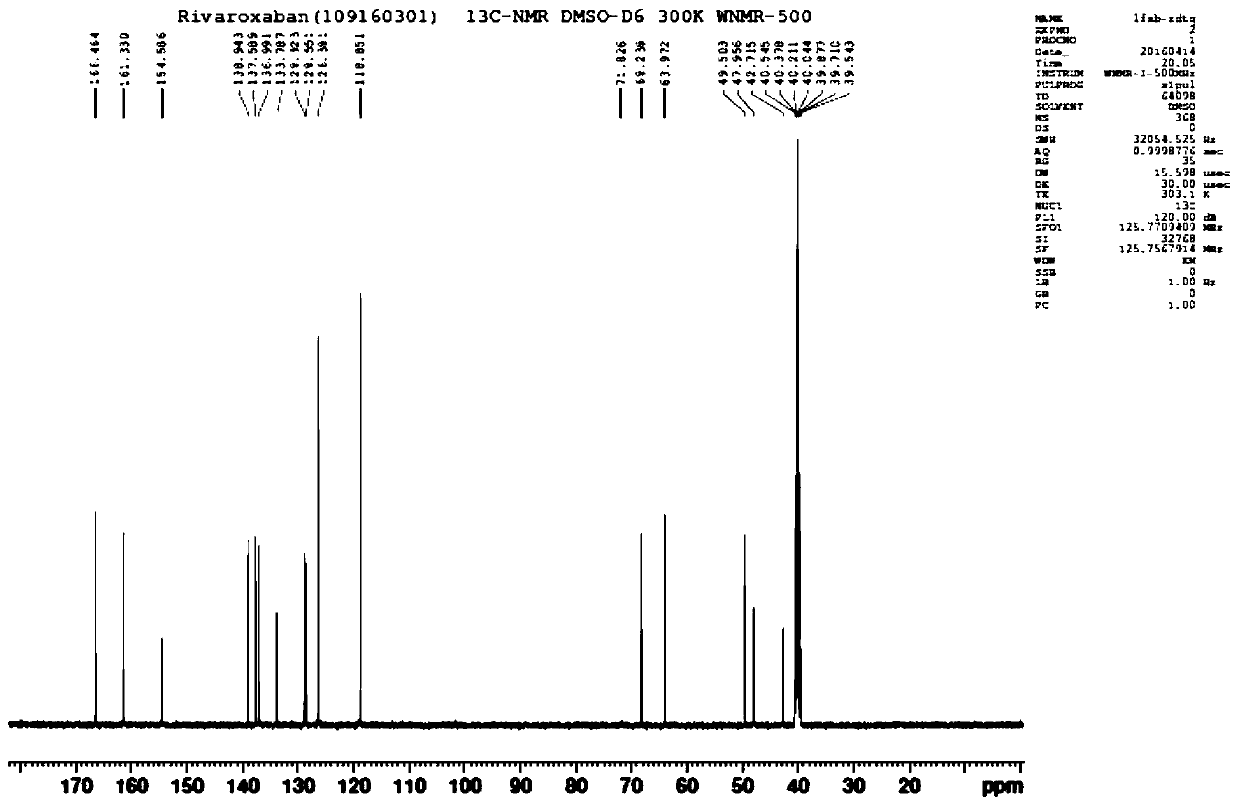
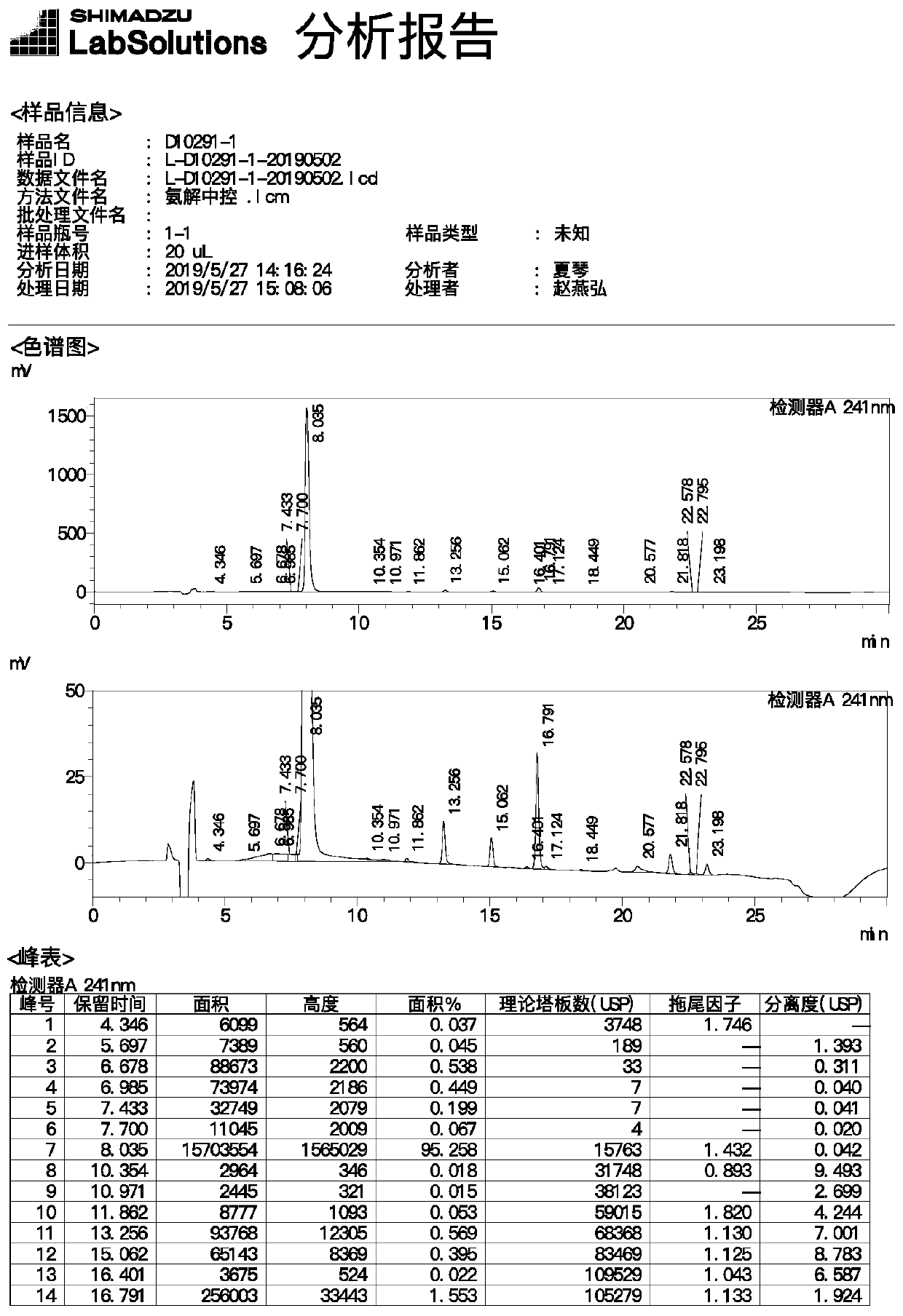
[0035] Drop into 4.0g 40% methylamine aqueous solution, 36g ethanol and 7.2g 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl) in 100ml four-necked reaction flask Phenyl]-5-oxazolidinyl]methyl]-1H-isoindole-1,3(2H)-dione, stir and heat up to 70°C, keep the temperature for 3 hours, image 3 The main raw material came out at 4.346 minutes, the reaction of the raw material was complete, the system was evaporated to dryness under reduced pressure, 40g of purified water and 2.2g of sodium carbonate were added, stirred to dissolve, and 13.4g of 30% 5-chlorothiophene-2-formyl chloride was added dropwise at 10°C Solution, insulation reaction 1 hour monitoring reaction completes, Figure 4 The main raw material peaked at 2.633 minutes, and the reaction of the raw material was complete. Add 50g of acetone and continue to stir for 0.5 hours, filter to obtain a white solid, rinse with 30g of purified water and 30g of acetone, and dry in vacuum to obtain 6.9g of dry product of rivaroxaban (yield ...
Embodiment 2
[0037] Drop into 4.0g 40% aqueous methylamine solution, 36g methanol and 7.2g 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl) in 100ml four-necked reaction flask Phenyl]-5-oxazolidinyl]methyl]-1H-isoindole-1,3(2H)-dione, stir and heat up to 70°C, keep warm for 3 hours, evaporate the system to dryness under reduced pressure, add 40g Purified water and 2.2g of sodium carbonate were stirred and dissolved, and 13.4g of 30% 5-chlorothiophene-2-formyl chloride toluene solution was added dropwise at 10°C, and kept for 1 hour to monitor the completion of the reaction, and 50g of acetone was added to continue stirring for 0.5 hours, and filtered to obtain white The solid was rinsed with 30 g of purified water and 30 g of acetone, respectively, and dried under vacuum to obtain 6.7 g of dry product of rivaroxaban (90.0% yield, 99.88% purity);
Embodiment 3
[0039]Drop into 4.0g 40% methylamine aqueous solution, 36g ethanol and 7.2g 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl) in 100ml four-necked reaction flask Phenyl]-5-oxazolidinyl]methyl]-1H-isoindole-1,3(2H)-dione, stir and heat up to 60°C, keep warm for 3 hours, evaporate the system to dryness under reduced pressure, add 40g Purified water and 2.2g of sodium carbonate were stirred and dissolved, and 13.4g of 30% 5-chlorothiophene-2-formyl chloride toluene solution was added dropwise at 10°C, and kept for 1 hour to monitor the completion of the reaction, and 50g of acetone was added to continue stirring for 0.5 hours, and filtered to obtain white The solid was rinsed with 30 g of purified water and 30 g of acetone, respectively, and dried under vacuum to obtain 6.8 g of dry product of rivaroxaban (91.3% yield, 99.83% purity);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com