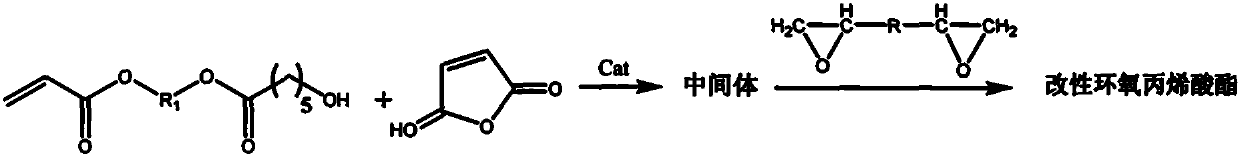
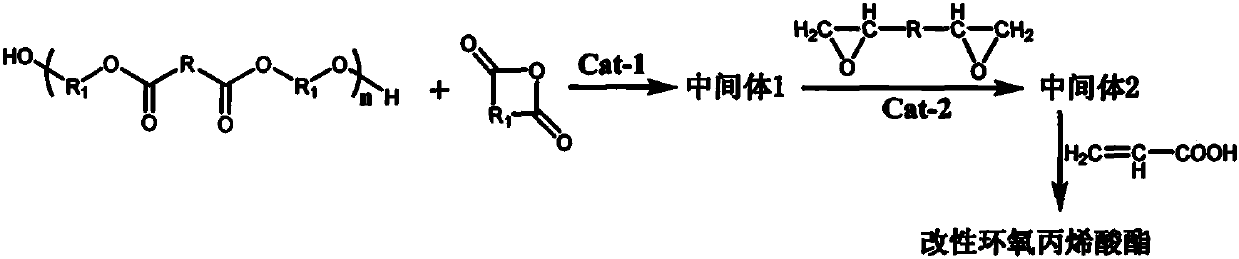
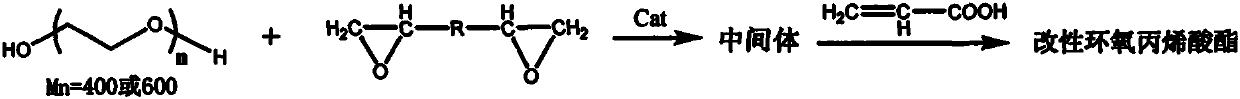Method for preparing modified propylene oxide ester
An epoxy acrylate and modified technology, applied in application, coating, ink and other directions, can solve the problem of high viscosity of bisphenol A epoxy acrylate finished product, limited flexibility of epoxy acrylate, and limited flexibility improvement and other problems, to achieve the effect of toughness advantage, strong designability, and wide adjustment range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add polyhexamethylene adipate 100g, succinic anhydride 40g, triethylamine 0.56g, N 2 Under protection, it was reacted at 70-80°C for 2h to obtain Intermediate 1.
[0024] (2) Add epoxy resin (epoxy equivalent 185-195g / eq, 248.3g) and tetraethylammonium bromide (2.1g) to intermediate 1, and react at 80-90°C for 2h to obtain the intermediate 2.
[0025] (3) Add acrylic acid (67.2g), hydroquinone (0.20g), and triphenylphosphine (1.0g) to intermediate 2, and react at 100-105°C for 4h to obtain modified epoxy acrylate a.
Embodiment 2
[0027] (1) Add polyhexamethylene adipate (100g), succinic anhydride (40g), triethylamine (0.56g), N 2 React at 70-80°C for 2h under protection to obtain Intermediate 1.
[0028] (2) Add epoxy resin (epoxy equivalent 185-195g / eq, 186.2g) and tetraethylammonium bromide (1.8g) to intermediate 1, and react at 80-90°C for 2h to obtain intermediate 2 .
[0029] (3) Add acrylic acid (43.2g), hydroquinone (0.18g), and triphenylphosphine (0.8g) to intermediate 2, and react at 100-105°C for 4h to obtain modified epoxy acrylate b.
Embodiment 3
[0031] (1) Add polyhexamethylene adipate (100g), succinic anhydride (40g), triethylamine (0.56g), N 2 Under protection, it was reacted at 70-80°C for 2h to obtain Intermediate 1.
[0032] (2) Add epoxy resin (epoxy equivalent 185-195g / eq, 149.0g) and tetraethylammonium bromide (1.5g) to intermediate 1, and react at 80-90°C for 2h to obtain intermediate 2 .
[0033] (3) Add acrylic acid (28.8g), hydroquinone (0.16g), and triphenylphosphine (0.8g) to intermediate 2, and react at 100-105°C for 4h to obtain modified epoxy acrylate c.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com