Adriamycin polyprodrug nano-micelle with reducibility response and preparation method and application thereof
A technology of nano-micelle and doxorubicin, which is applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of high toxic side effects, poor water solubility, and short half-life of doxorubicin, etc. problem, achieve the effect of reducing drug toxicity, improving water solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
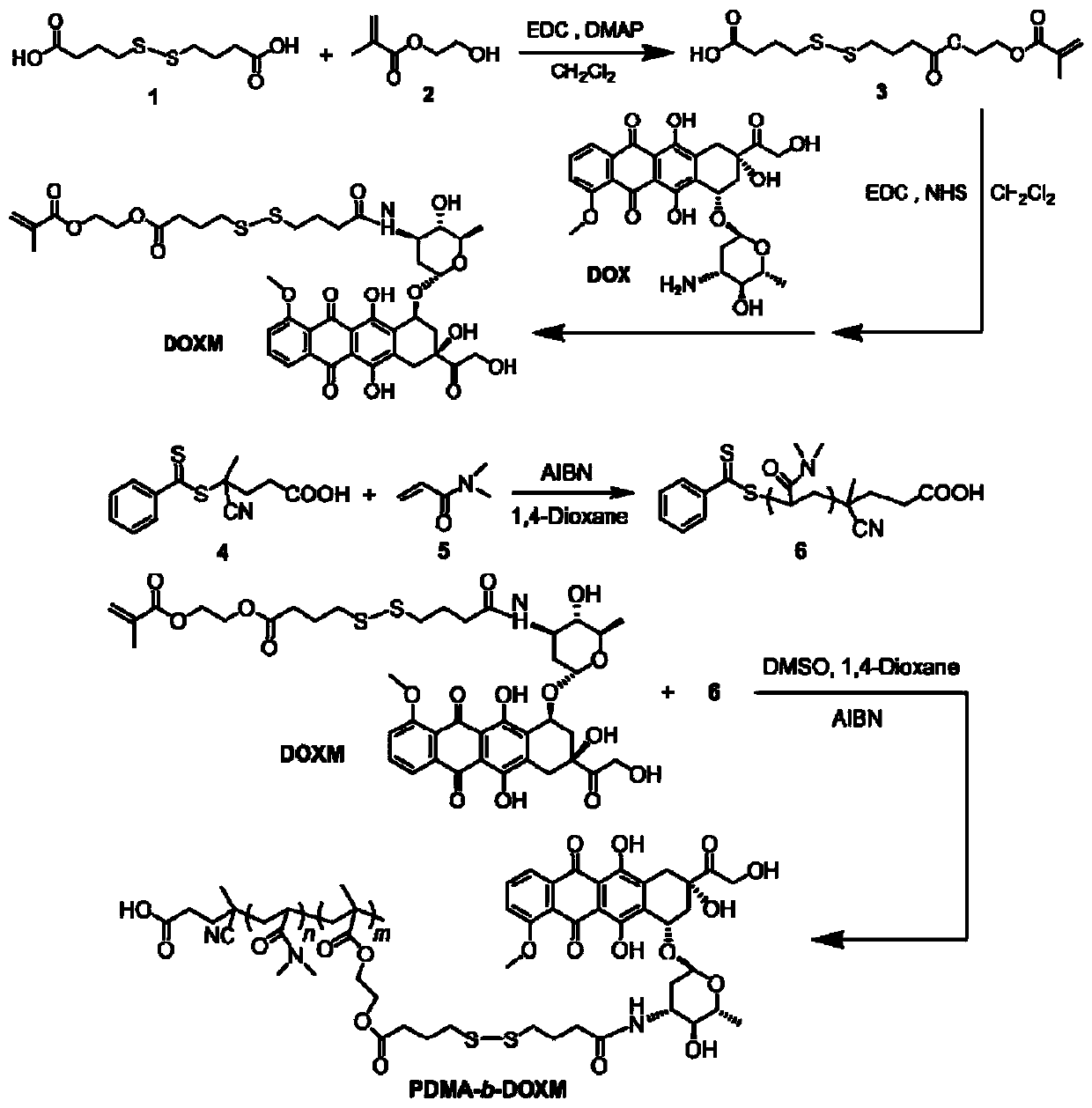
[0047] Example 1: Single-ended modification of 4,4'-dithiodibutyric acid
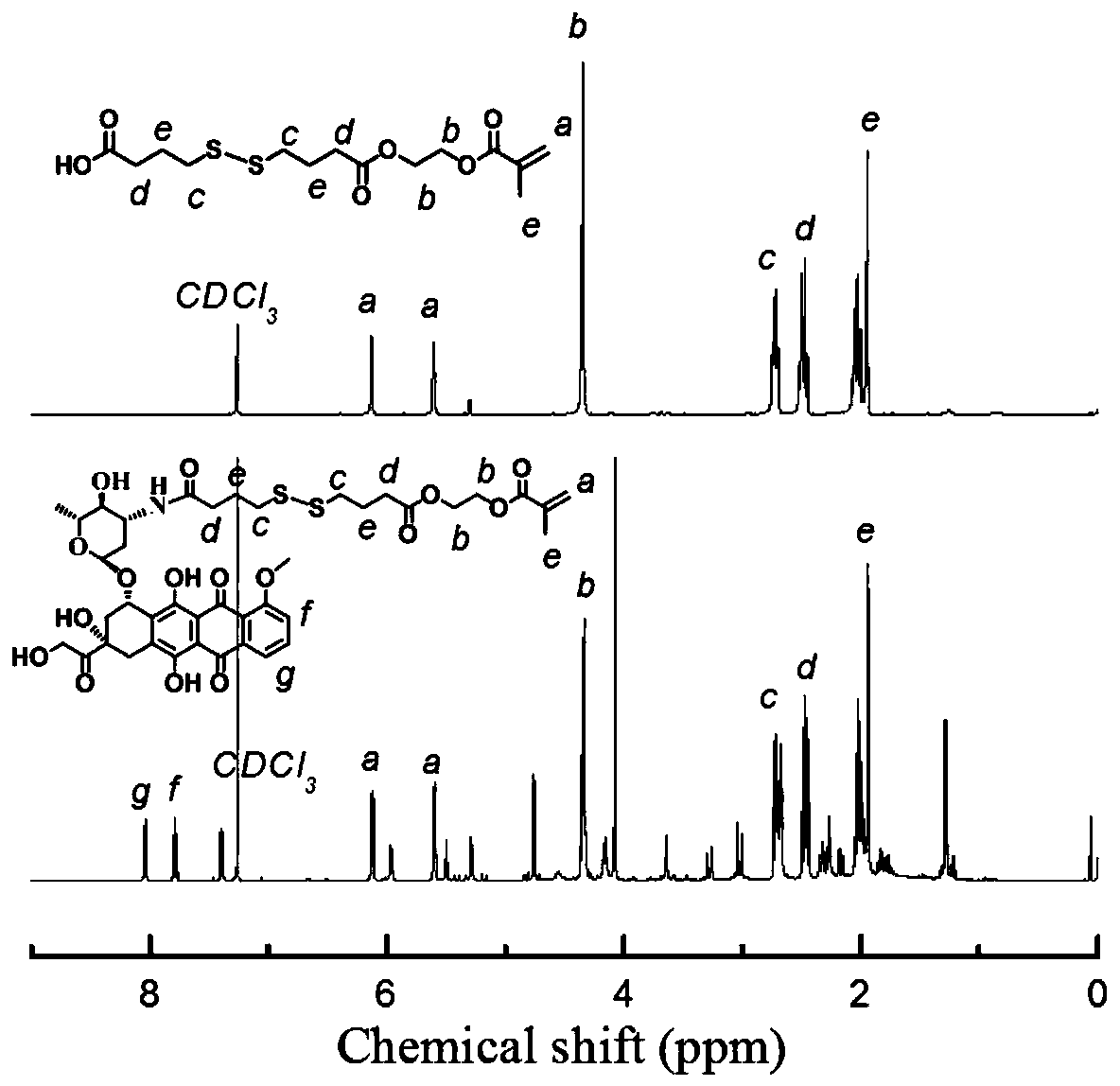
[0048] In a 250mL double-necked round bottom flask, weigh 4,4'-dithiodibutyric acid (5g, 20.98mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt EDC (8.04g, 41.96mmol), DMAP (5.13g, 41.96mmol) and 100mL of dry dichloromethane were cooled in an ice-water bath and stirred with a magnet until completely dissolved. Return to room temperature and continue stirring for 4 h to fully dissolve the mixture, then add 2-hydroxyethyl methacrylate HEMA (2.49 g, 19.11 mmol), and stir overnight at room temperature. After 24 h, the reaction was stopped, and the solvent was removed by rotary evaporation. The crude product was separated and purified by silica gel column chromatography, and eluted with petroleum ether:dichloromethane=1:1 and dichloromethane to obtain a yellow oily liquid (4.19g, yield: 56%).
Embodiment 2
[0049] Example 2: Preparation of reductively responsive doxorubicin prodrug monomer DOXM
[0050] Doxorubicin hydrochloride is first desalted. In a 250 mL round bottom flask, weigh doxorubicin hydrochloride (40 mg, 6.9 mmol) and disperse it in 150 mL of dry dichloromethane, and add 200 mL of triethylamine (1 mg DOX·HCl is added to 5 mL of triethylamine). Stir at room temperature until doxorubicin hydrochloride is completely dissolved, concentrate the liquid to 30mL by rotary evaporation, collect the filtrate after filtration for use, weigh the product in Example 1 (24.18mg, 6.9mmol), 1-ethyl-(3-di Methylaminopropyl)carbodiimide hydrochloride EDC (2.65 mg, 13.8 mmol), N-hydroxysuccinimide NHS (1.59 mg, 13.8 mmol) were dissolved in 10 mL of dry dichloromethane. After stirring overnight at room temperature for 24 hours, the desalted DOX solution was added to continue the reaction for 24 hours. After the reaction, extract three times with deionized water and saturated NaCl aqueo...
Embodiment 3
[0051] Embodiment 3: the preparation of poly(N,N-dimethylacrylamide) PDMA macromolecular chain transfer agent
[0052] The chain transfer agent 4-cyano-4-(phenylthioformylthio)valeric acid (compound 4) (15 mg, 0.054 mmol), N,N-dimethylacrylamide (compound 5) (DMA, 319.4 mg, 3.222 mmol) and azobisisobutyronitrile (AIBN, 1.77 mg, 0.011 mmol) were dissolved in 1 mL of 1,4-dioxane in an ampoule. Freeze the ampoule bottle in liquid nitrogen and use an oil pump to pump air, then seal the ampoule bottle, return to room temperature to melt the reaction mixture, and then freeze and pump air again. Repeat the freeze-thaw cycle three times to make the reaction environment as free as possible. Air, in a state of vacuum. Then seal again, stir and react at 70°C for 13 hours, then stop the polymerization reaction with liquid nitrogen, open the reaction bottle, precipitate the reaction mixture in glacial ether, centrifuge, redissolve in dichloromethane and precipitate with a large amount of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
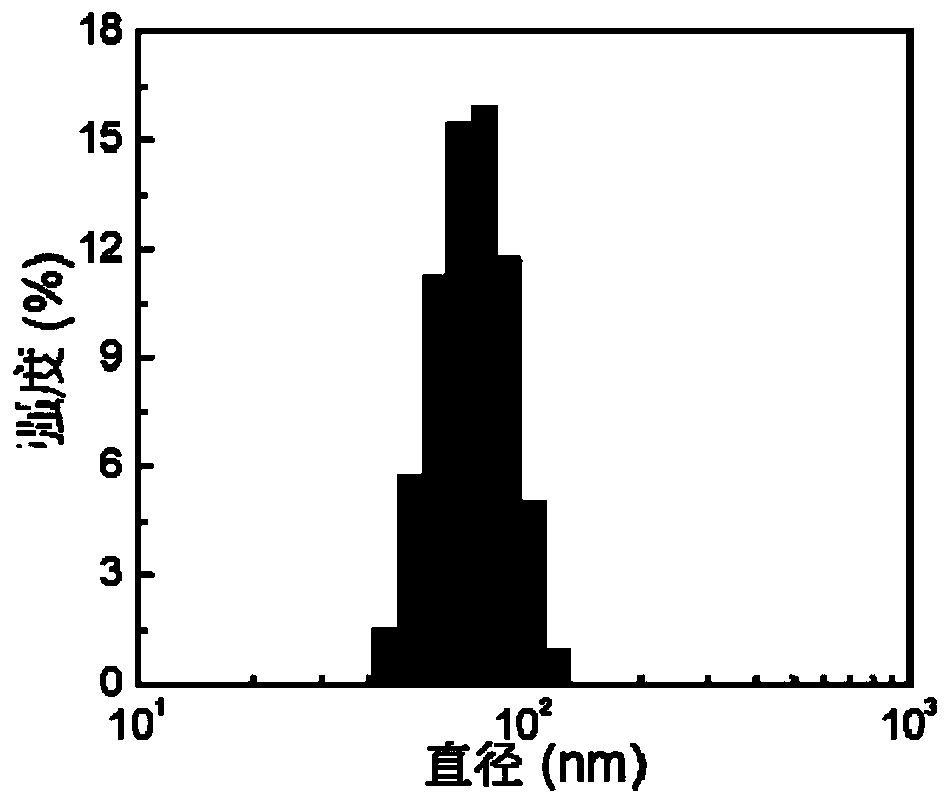
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com