Universal vaccine immunopotentiator
An immune enhancer, general-purpose technology, applied in the field of biotechnology genetic engineering, can solve problems such as few research and development reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Construction of Escherichia coli expression vector and expression strain
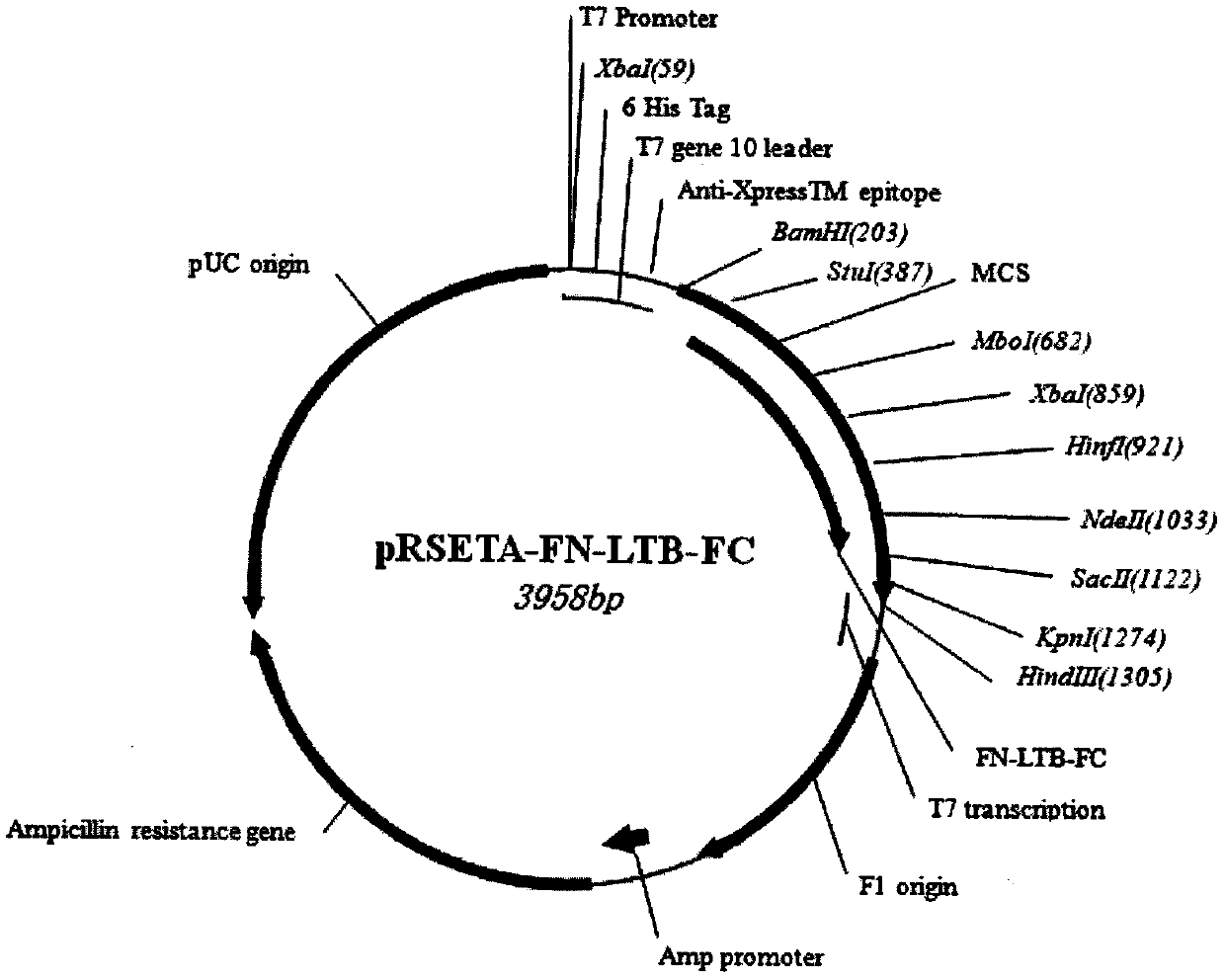
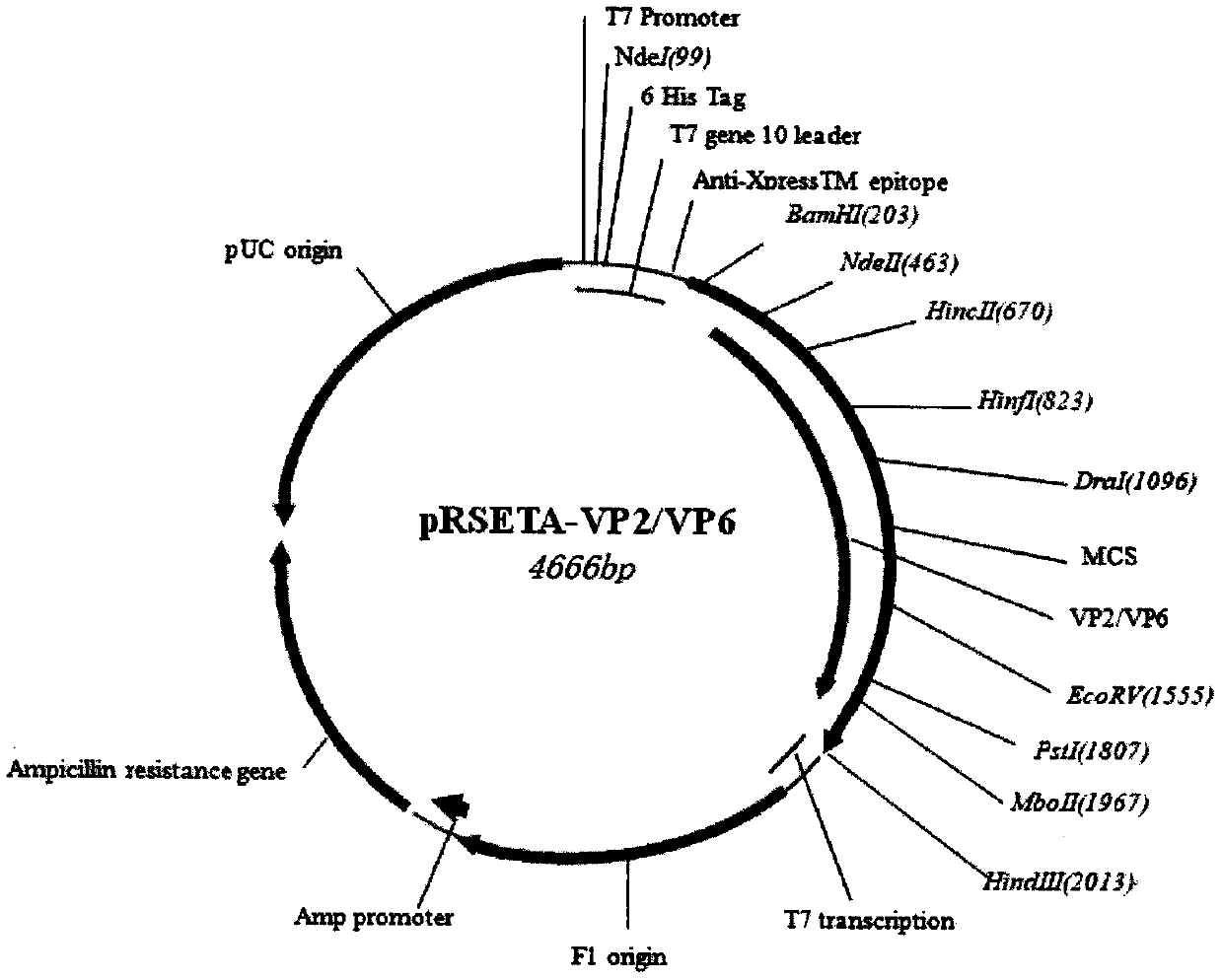
[0027] 1 Send the designed polypeptide encoding nucleotides (see the sequence table SEQ1 and SEQ3) to Shanghai Handsome Biotechnology Co., Ltd. for synthesis, and the two ends of the nucleotide fragments are respectively designed with BamH I (5' end) and HindIII (3' end) restrictions After the fragments were synthesized, they were respectively cloned into the pMD18T vector, and sequence determination confirmed that the inserted gene fragments were consistent with the designed sequence. The recombinant plasmids were named pMD18T-FN-LTB-FC and pMD18T-VP2 / VP6, respectively. Digest the plasmid with the corresponding restriction endonuclease. The expression vector of E. coli is the pRSETA plasmid from Invitrogen Company, which is also treated with the same restriction endonuclease. Digestion conditions: 10 μl reaction system, add 2 μl plasmid into the system , 5 activity units of restrictio...
Embodiment 3
[0031] Embodiment 3 Fermentation, purification and preparation of engineering bacteria
[0032] 1 Take the production strains pRSETA-FN-LTB-FC / BL21 (DE3, Plys) and pRSETA-VP2 / VP6 / BL21 (DE3, Plys) from fermentation, and inoculate them in 2mL LB liquid medium (containing 100 μg / mL ampicillin ), 37°C, 200rpm shaking culture for 12 hours to activate the strain. The inoculum was then placed in shake flasks at an inoculum size of 1:100, cultured with shaking at 37°C until OD600=3, and then inoculated into a fermenter at a ratio of 10%. The medium used for fermentation is a semi-synthetic medium prepared with distilled water. Calibrate dissolved oxygen and pH electrodes, turn on the tank to stir at 300 rpm, and sterilize the tank on-line. When the temperature of the culture solution in the tank drops to 37.0°C, calibrate the zero point of pH and dissolved oxygen (OD). The fermentation temperature was 37.0±0.1°C, the dissolved oxygen was controlled at about 40%, and the pH was contr...
Embodiment 4
[0036] Example 4 Safety Test of Universal Vaccine Immunopotentiator
[0037] 1 material
[0038] 1.1 Vaccine: The emulsified emulsion of the immune enhancer is provided by Qingdao Mingqin Biological Research and Development Center, and the batch numbers are 201701, 201702, and 201703.
[0039] 1.2 Experimental animals: 20-25g Balb / C mice were purchased from Beijing Huafukang.
[0040] 2 methods
[0041] Balb / C mice, 17. Each batch of vaccine immunized 5 mice, totally 3 batches, and immunized 15 mice. The immunization method was subcutaneous injection, and the control group was immunized with physiological saline emulsion in the same way. The health status of the mice was observed continuously for 10 days after immunization.
[0042] 3 results
[0043] The results are shown in Table 1. After immunization, the appetite, spirit and health status of all mice were normal, consistent with the control group, and no death occurred. It can be seen that the vaccine immune enhancer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com