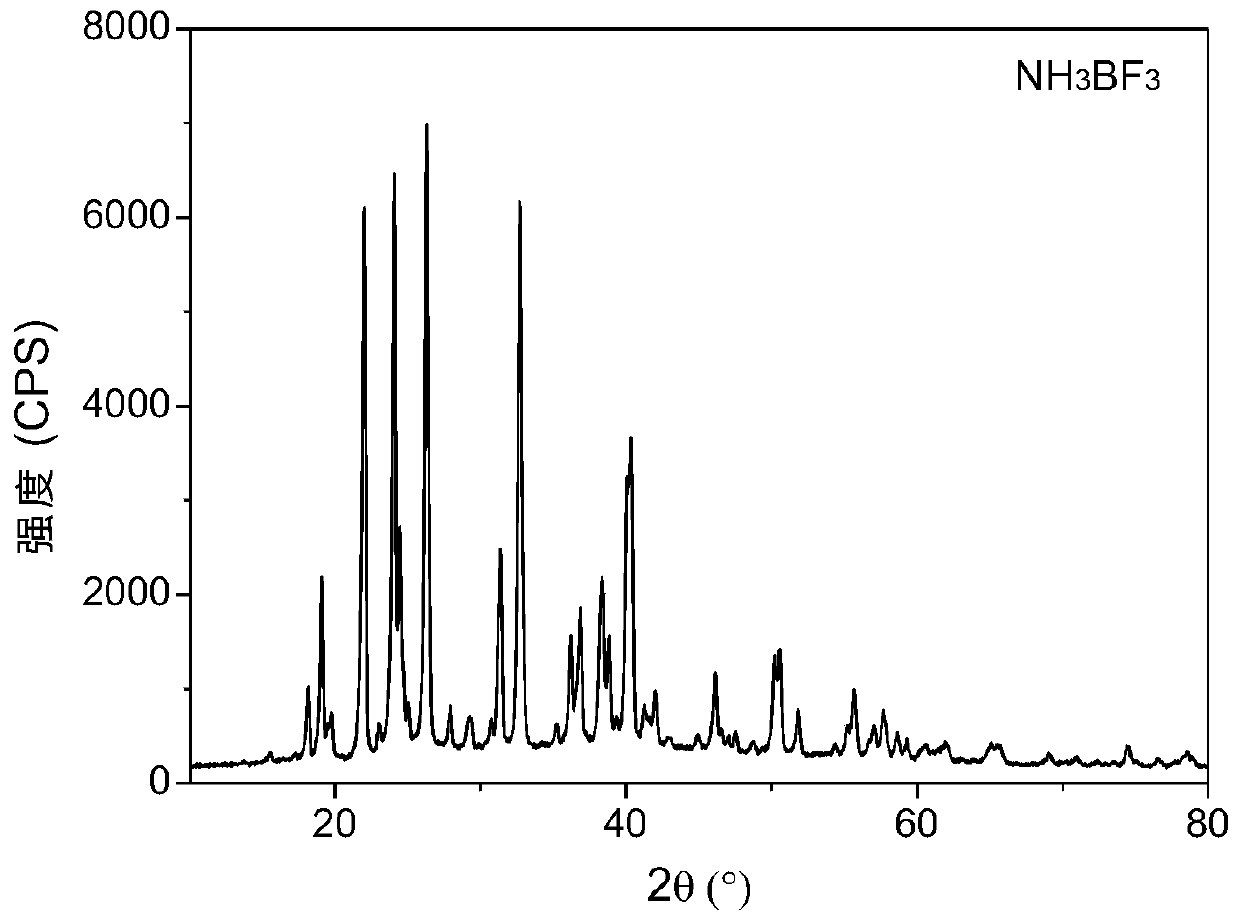
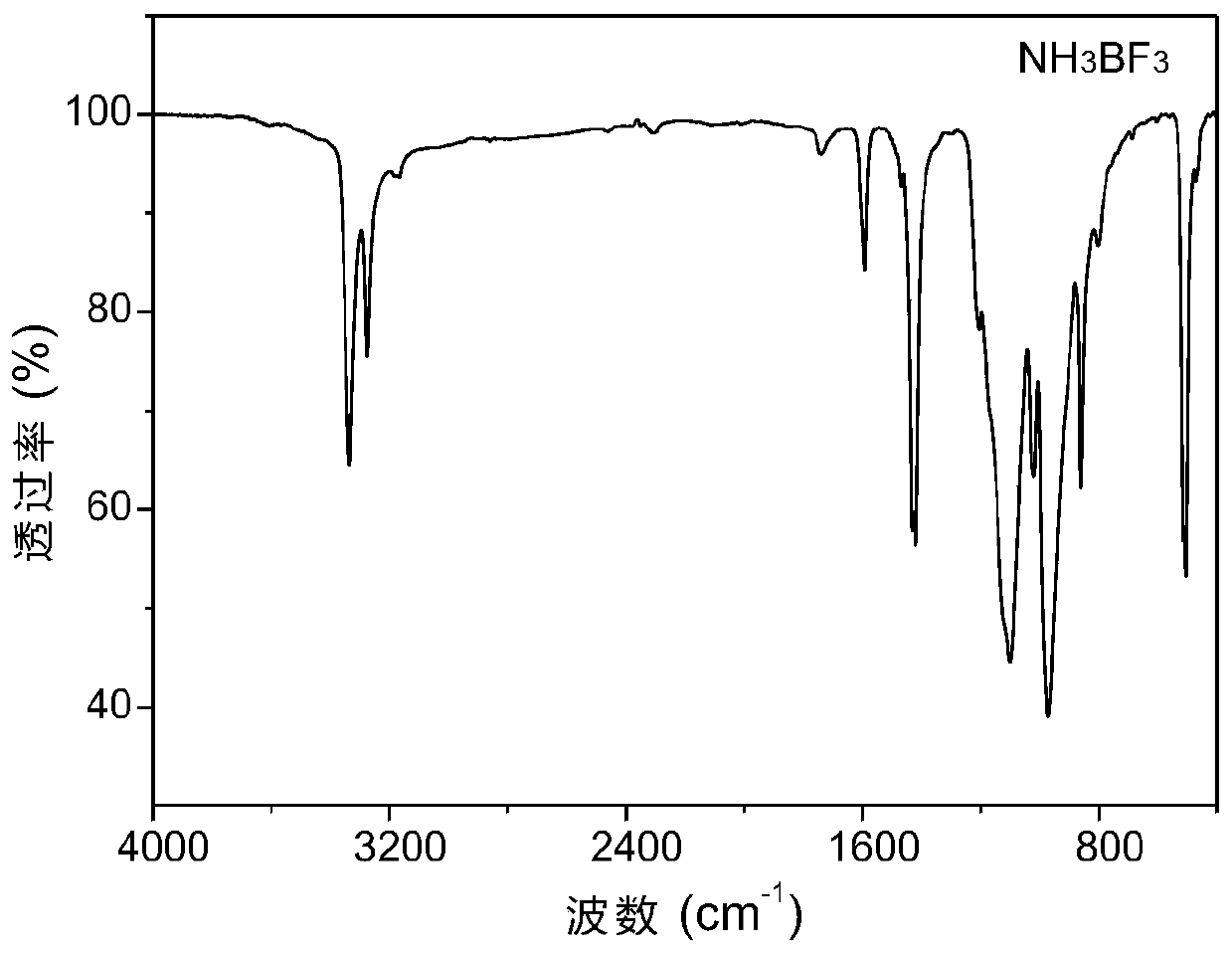
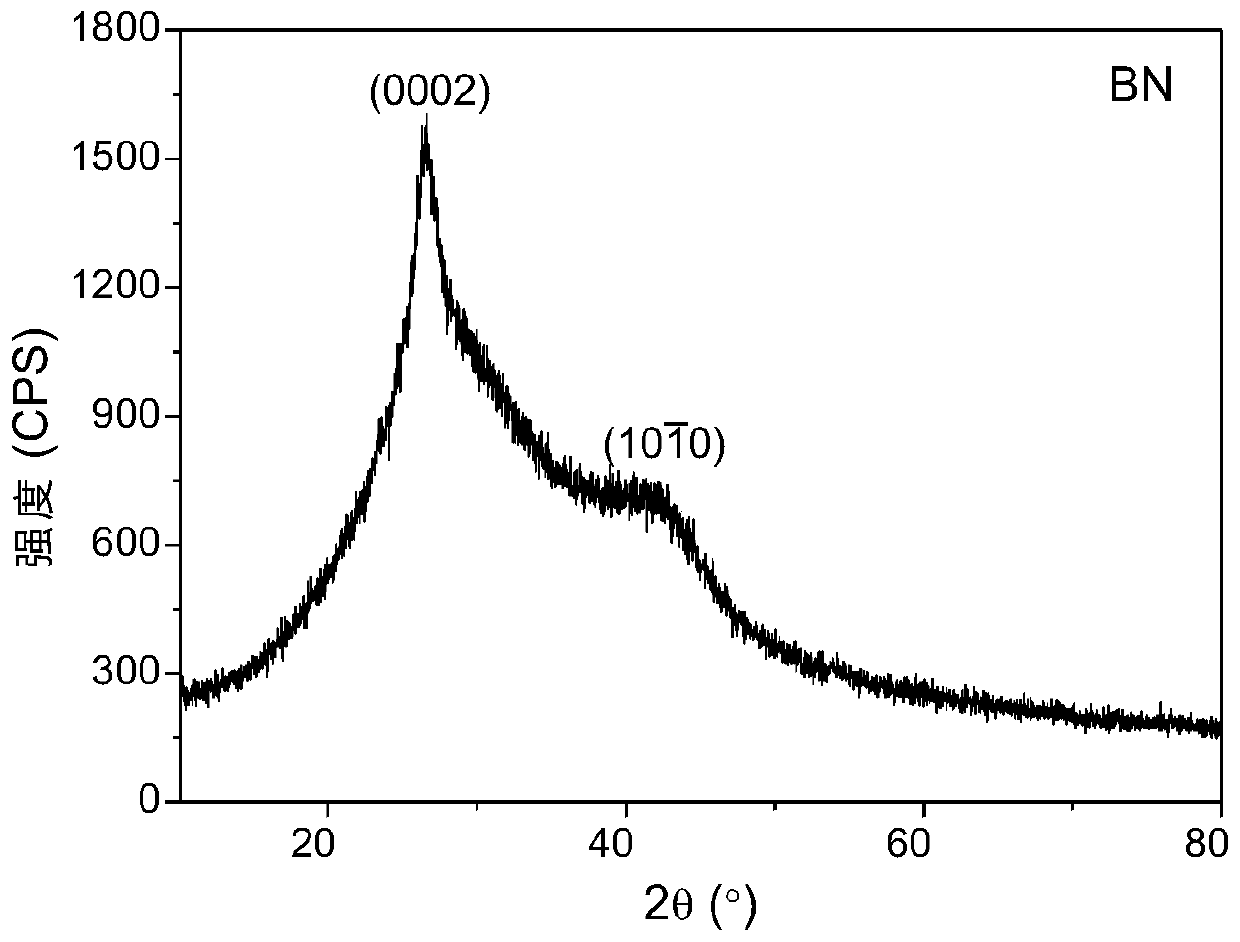
Method for synthesizing boron nitride and ammonium fluoborate at low temperature
A technology for ammonium fluoroborate and boron nitride, which is applied in the field of synchronous synthesis of boron nitride and ammonium fluoroborate at low temperature, which can solve the problems of high requirements for experimental equipment and high risk factors, achieve high purity, avoid high-temperature processes, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Continuously feed ammonia gas (flow rate: 100ml / min) into a three-necked bottle filled with 50ml of boron trifluoride diethyl ether, ice-water bath and constant stirring, the reaction process exothermic and accompanied by white smoke generation, until the liquid Complete reaction transforms into white boron trifluoride ammonium powder;
[0036] (2) Put the white boron trifluoride ammonium powder obtained by gas-liquid reaction in step (1) into a 100ml beaker, treat it at 125°C for 30 minutes, and let it cool down to room temperature naturally to obtain ammonium fluoroborate and low crystallinity Mixed powder of boron nitride;
[0037] (3) Dissolving the mixed powder in step (2) in excess deionized water, dissolving ammonium fluoroborate completely, filtering and washing repeatedly, collecting wet boron nitride solid and clarified filtrate;
[0038] (4) The wet boron nitride solid and the filtrate obtained in step (3) are freeze-dried respectively to obtain phase-se...
Embodiment 2、3、4
[0042] Change the ammonia gas flow rate in the step (1) of embodiment 1 into 50ml / min, 200ml / min, 300ml / min respectively, and other operations are all the same as in embodiment 1, and the properties of the obtained precursor and the two products are the same Example 1.
Embodiment 5、6、7、8、9
[0044] Change the amount of boron trifluoride ether in embodiment 1, 2, 3, and 4 steps (1) to 10ml, 100ml, 150ml, 200ml, 250ml, and other operations are the same as in Example 1, and the obtained precursor The properties of body and two products are the same as example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com