Method for continuously preparing bentazone
A bentazon and flow rate control technology, applied in the direction of organic chemistry, can solve the problems of equipment corrosion, easily lead to material flushing, slow reaction speed, etc., and achieve the effects of high safety, improved production efficiency, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
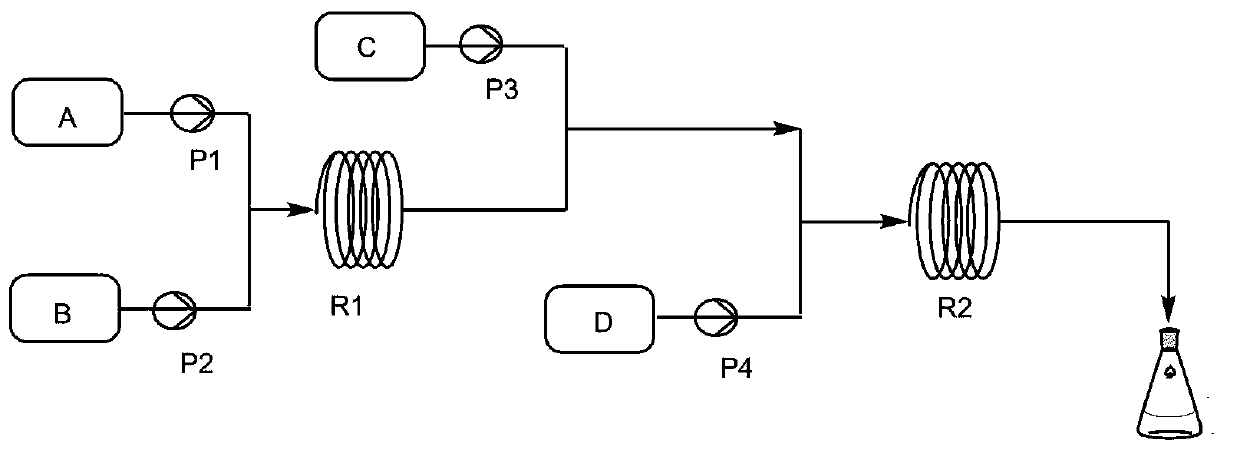
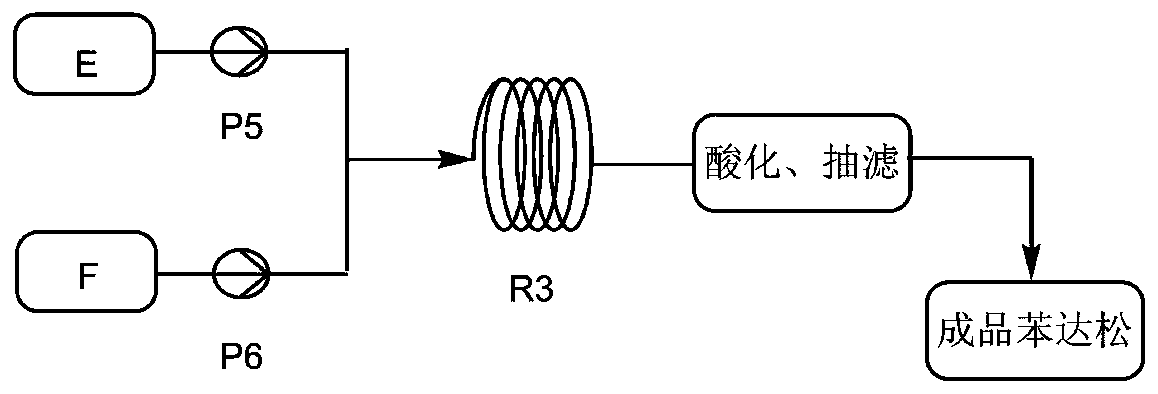
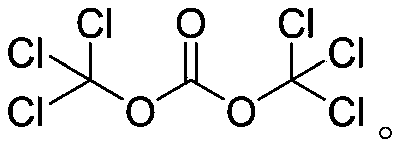
[0031] Such as figure 1 and figure 2 As shown, isopropylamine 22g and acid-binding agent triethylamine 145g are miscible with solvent dichloroethane 200g as material A, chlorosulfonic acid 38.2g as material B, methyl anthranilate 45g as material C, BTC53. 06 g and solvent dichloroethane 250g are miscible as material D, material A and material B are transported into tubular reactor R1 through infusion pump P1 and infusion pump P2 respectively, and the temperature of tubular reactor R1 is controlled by a water bath to 0°C. By setting the flow rate of the pump to control the residence time of the material in the tubular reactor R1 to 0.4min, the sulfonylation reaction is carried out in the tubular reactor R1 to generate isopropylamine sulfonic acid, and then the isopropylamine sulfonic acid is transported with the infusion pump P3 The material C is mixed, and the mixed material enters the tubular reactor R2 together with the material D delivered by the infusion pump P4. The tem...
Embodiment 2
[0033] Such as figure 1 and figure 2As shown, 24.66g of isopropylamine and 145g of acid-binding agent triethylamine are miscible with 200g of solvent dichloroethane as material A, 45.14g of chlorosulfonic acid as material B, 45g of methyl anthranilate as material C, BTC88 .43 g and solvent dichloroethane 250g are miscible as material D, material A and material B are transported into tubular reactor R1 through infusion pump P1 and infusion pump P2 respectively, and the temperature of tubular reactor R1 is controlled by a water bath to be 15°C , the residence time of the material in the tubular reactor R1 is controlled to be 1.0min by setting the pump flow rate, and the sulfonylation reaction is carried out in the tubular reactor R1 to generate isopropylamine sulfonic acid, and then the isopropylamine sulfonic acid is mixed with the infusion pump P3 The transported material C is mixed, and the mixed material enters the tubular reactor R2 together with the material D transporte...
Embodiment 3
[0035] like figure 1 and figure 2 As shown, 29.95g of isopropylamine and 145g of acid-binding agent triethylamine are miscible with 200g of solvent dichloroethane as material A, 55.56g of chlorosulfonic acid as material B, 45g of methyl anthranilate as material C, BTC123 .8 g and solvent dichloroethane 250g are miscible as material D, material A and material B are transported into tubular reactor R1 through infusion pump P1 and infusion pump P2 respectively, and the temperature of tubular reactor R1 is controlled by a water bath to be 30°C , by setting the pump flow rate to control the residence time of the material in the tubular reactor R1 to be 1.5min, the sulfonylation reaction is carried out in the tubular reactor R1 to generate isopropylamine sulfonic acid, and then the isopropylamine sulfonic acid is mixed with the infusion pump P3 The transported material C is mixed, and the mixed material enters the tubular reactor R2 together with the material D transported by the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com