Chimonin-2, 3-diketone and preparation method and medical application thereof
A compound and the obtained technology are applied in organic chemistry, antiviral agents, etc., and can solve the problems of anti-influenza virus, which are rarely reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 compound
[0037] Take the dried waxberry leaves, put them into an extraction tank, extract three times with 95%, 87%, and 80% ethanol, each time for 50 minutes, combine and concentrate the extracts three times, reclaim the ethanol, and obtain the total extract . Take the total extract, disperse it with warm water, carry out "acid extraction" with 2% hydrochloric acid, and extract it several times until the alkaloid reaction in the extract is negative, and the part soluble in acid water and the part insoluble in acid are obtained. The water part (non-alkaloid part), filter the acid water part and the insoluble part in acid water, take the alkaloid extract of the acid water part, carry out "alkali precipitation" with alkali, adjust the extract of the acid water part with concentrated ammonia water To pH=9, extract with chloroform for 3-4 times until the alkaloid color reaction is negative, recover chloroform, and obtain the extract of pH=...
Embodiment 2
[0040] The chemical structure determination of embodiment 2 compound
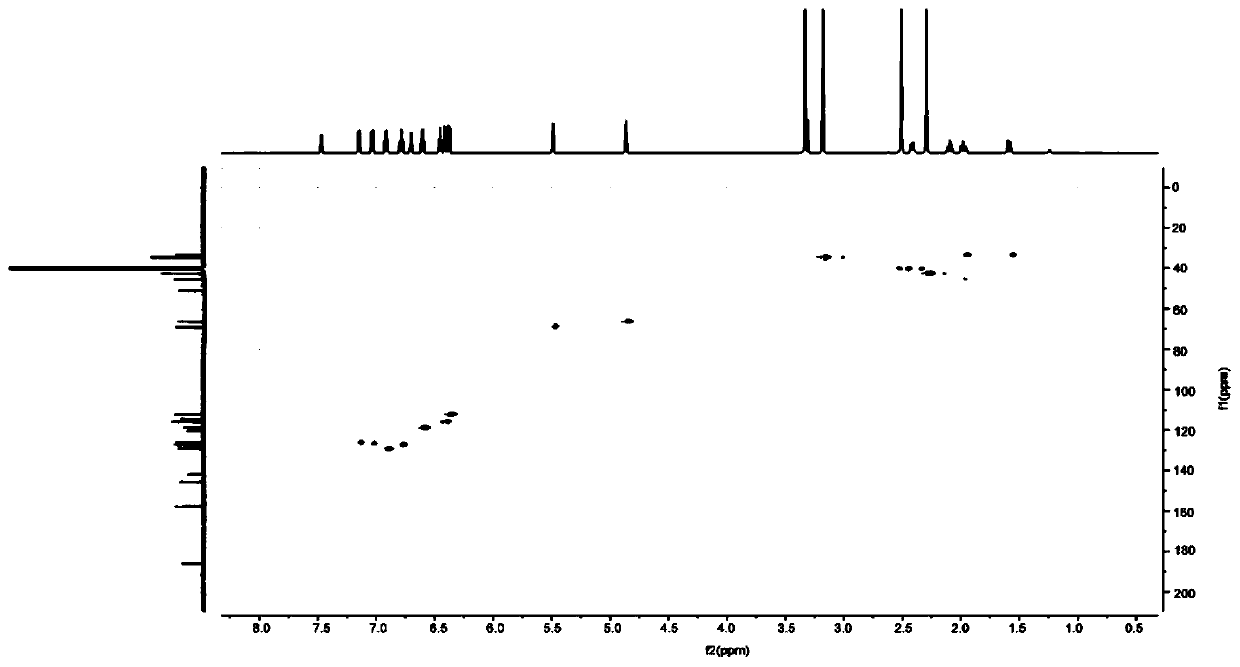
[0041] Structure Determination JASCO V650 UV spectrophotometer was used to measure UV spectrum, Brukeravance Ⅲ HD 600HZ NMR instrument was used to record spectrum in DMSO solvent, and mass spectrum was measured with AB SCIEX Triple TOF 5600+ high-resolution mass spectrometer.
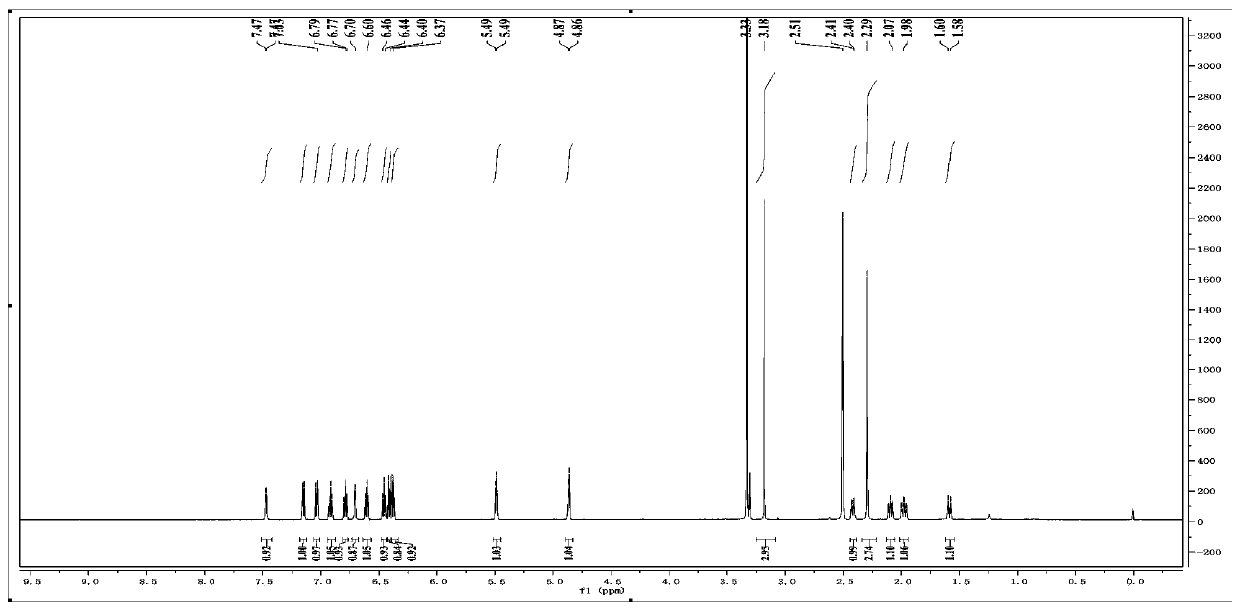
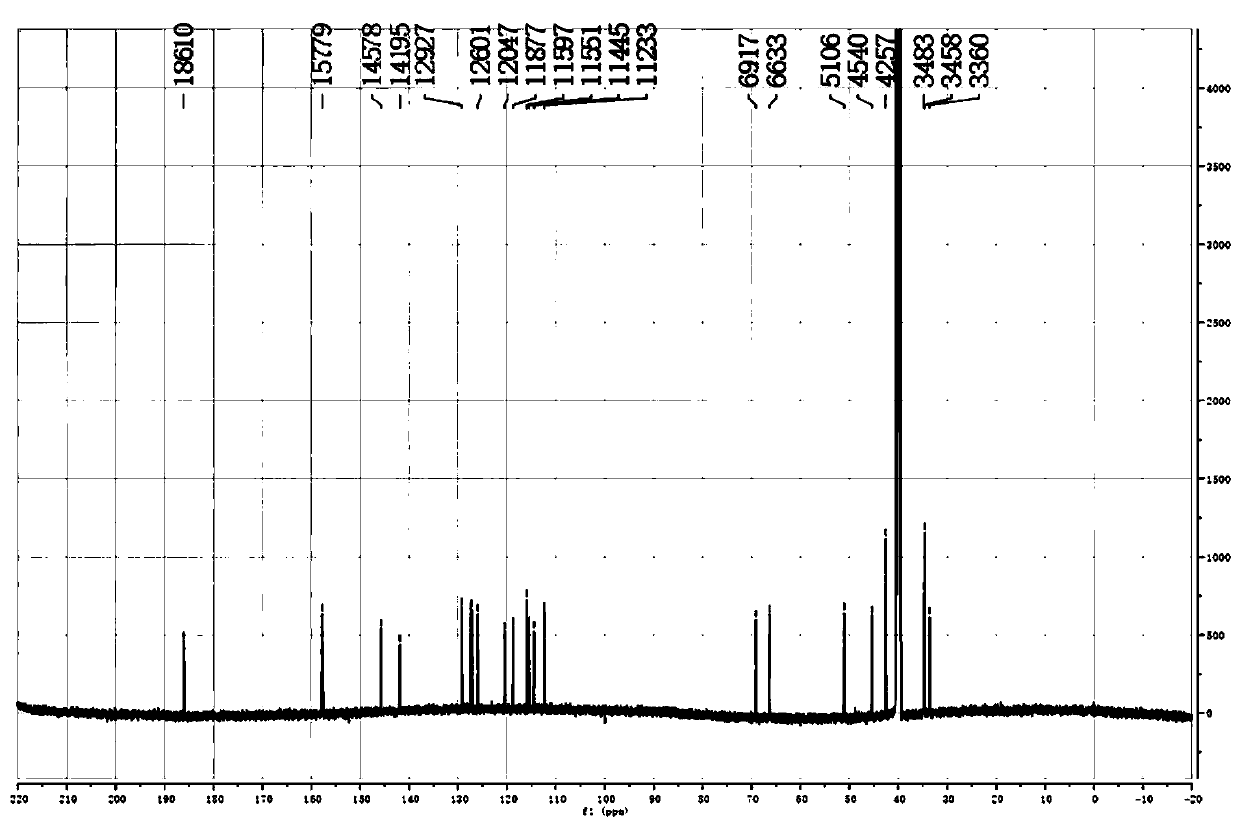
[0042] Physical and chemical properties of the compound Colorless transparent needle-like crystals (methanol), the thin-layer plate has dark spots under the irradiation of a UV lamp with a wavelength of 245nm, and no fluorescence under the irradiation of 365nm. UV (CH 3 OH) λmax=206nm, HR-ESI-MS m / z 375.1817[M+H] + , 749.3548[2M+H] + , the molecular formula is C 22 h 22 N 4 o 2 ; 1 H-NMR (600MHz, DMSO-d6) δ: 7.47 (1H, d, J = 4.2, 7a-N), 7.15 (1H, d, J = 7.2, H-4'), 7.04 (1H, d, J =7.8, H-4), 6.92 (1H, t, J=7.6, H-6), 6.79 (1H, t, J=7.6, H-6'), 6.70 (1H, d, J=4.5, 7a '-N), 6.60 (1H, t, J=7.5, H-5), 6.45 (1H, t, J=7.1, H-5'), ...
Embodiment 3
[0045] The cell experiment of embodiment 3 compound anti-influenza A virus H1N1 effect
[0046] Experimental material: virus strain: type A influenza virus H1N1 subtype (A / PuertoRico / 8 / 1934).
[0047] Cell model: Dog kidney cells MDCK (ATCC CCL-34) Culture conditions: DMEM+10% fetal bovine serum, 37°C, 5% CO 2 .
[0048] Experimental main instruments and reagents: (Promega, substrate product number G755B, lot number 0000174838; diluent product number G756B, lot number 0000173700) kit, biological safety cabinet (Harbin Donglian Electronic Technology Development Co., Ltd. BSC-1360-LIIB2), EnSpire (Perkin-Elmer) multifunctional enzyme Standard instrument, MUNANA Sigma-Aldrich, DMEM medium (Gibco, product number 12800-017, batch number 1791920), fetal bovine serum (FBS, Biological Industries, product number 04-001-1ACS, batch number 1534372), compound mycetine-2,3 diketone.
[0049] experimental method:
[0050] Preparation of MUNANA solution
[0051] Preparation of MUNANA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com