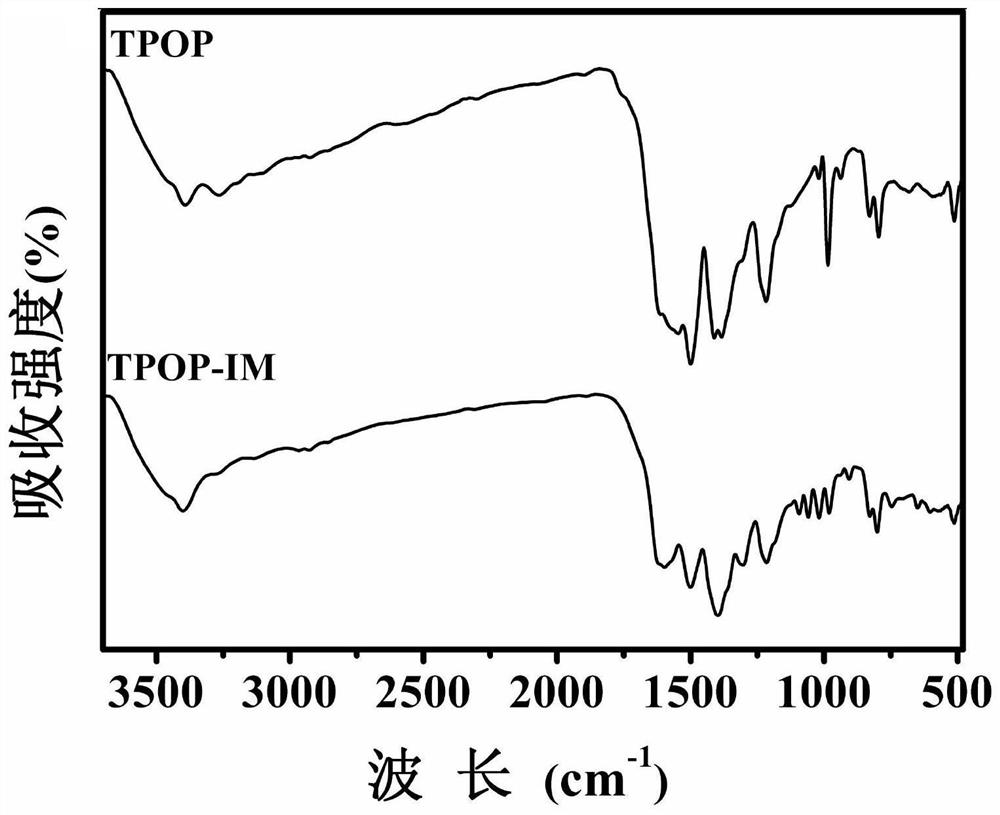
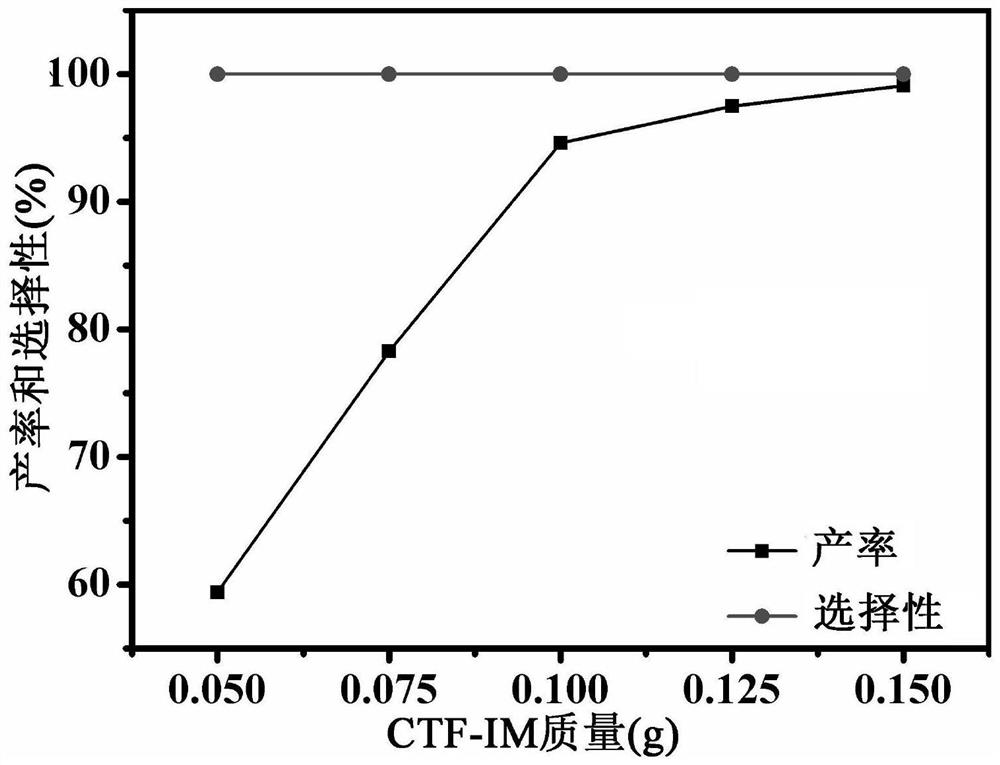
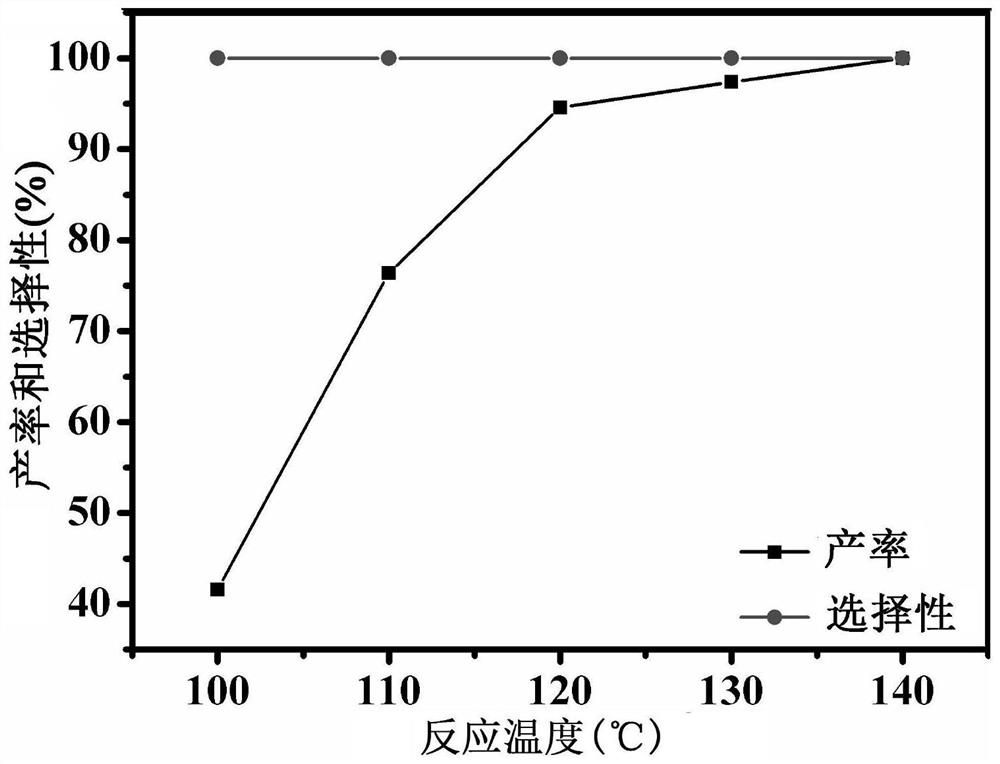
A kind of imidazole functionalized covalent triazine framework material and its preparation method and application
A covalent triazine and framework material technology, applied in chemical instruments and methods, carbon dioxide or inorganic carbonate preparation, catalytic reactions, etc., can solve problems such as poor catalytic performance, achieve high-efficiency catalysis, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides the preparation method of the imidazole-functionalized covalent triazine frame material described in the technical scheme, comprising the following steps:
[0033] Mix the covalent triazine framework, N,N'-carbonyldiimidazole and furan organic solvent for addition reaction to obtain imidazole functionalized covalent triazine framework material;
[0034] The repeating structural unit having the structure shown in formula II of the covalent triazine framework:
[0035]
[0036] In Formula II, the wavy line represents a repeating basic unit.
[0037] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
[0038] In the present invention, the preparation method of the covalent triazine framework (CTF) at room temperature preferably includes the following steps: mixing p-phenylenediamine, inorganic alkaline reagent, water, cyanuric chlori...
Embodiment 1
[0057] P-phenylenediamine (0.81g), sodium carbonate (0.3175g) and deionized water (10mL) were mixed to obtain a p-phenylenediamine solution; cyanuric chloride (0.3688g) and acetone (25mL) were mixed to obtain three Polycyanuric chloride solution: adding the p-phenylenediamine solution dropwise to the cyanuric chloride solution, reacting at room temperature for 8 hours, then suction-filtering the obtained reaction system, washing the obtained solid product with water three times, acetone Washed 3 times and washed 3 times with methanol, placed in a vacuum drying oven at 90° C. for 10 h in vacuum to obtain a covalent triazine framework (CTF).
[0058] A covalent triazine framework (0.75 g) was mixed with THF (25 mL) to obtain a covalent triazine framework solution; N,N'-carbonyldiimidazole (1.6215 g) was mixed with THF (20 mL) to obtain N,N' -carbonyldiimidazole solution; mix the covalent triazine framework solution and N,N'-carbonyldiimidazole solution, reflux reaction at 65°C f...
Embodiment 2
[0064] P-phenylenediamine (0.324g), sodium carbonate (0.3175g) and deionized water (10mL) were mixed to obtain a p-phenylenediamine solution; cyanuric chloride (0.3688g) and acetone (20mL) were mixed to obtain three Polycyanuric chloride solution: adding the p-phenylenediamine solution dropwise to the cyanuric chloride solution, reacting at room temperature for 12 hours, then suction-filtering the obtained reaction system, washing the obtained solid product with water three times, acetone Washed three times and washed three times with methanol, placed in a vacuum drying oven at 80° C. for 12 hours in vacuum to obtain a covalent triazine framework.
[0065] Mix the covalent triazine framework (0.75 g) and tetrahydrofuran (25 mL) to obtain a covalent triazine framework solution; mix N,N'-carbonyldiimidazole (1.2 g) and tetrahydrofuran (20 mL) to obtain N,N' -carbonyldiimidazole solution; mix the covalent triazine framework solution and N,N'-carbonyldiimidazole solution, reflux r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com