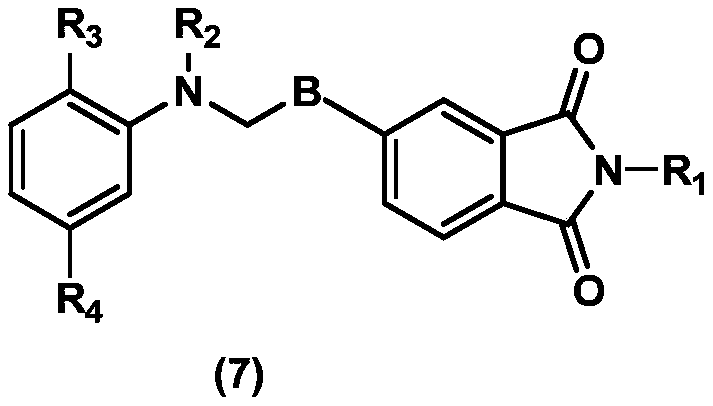
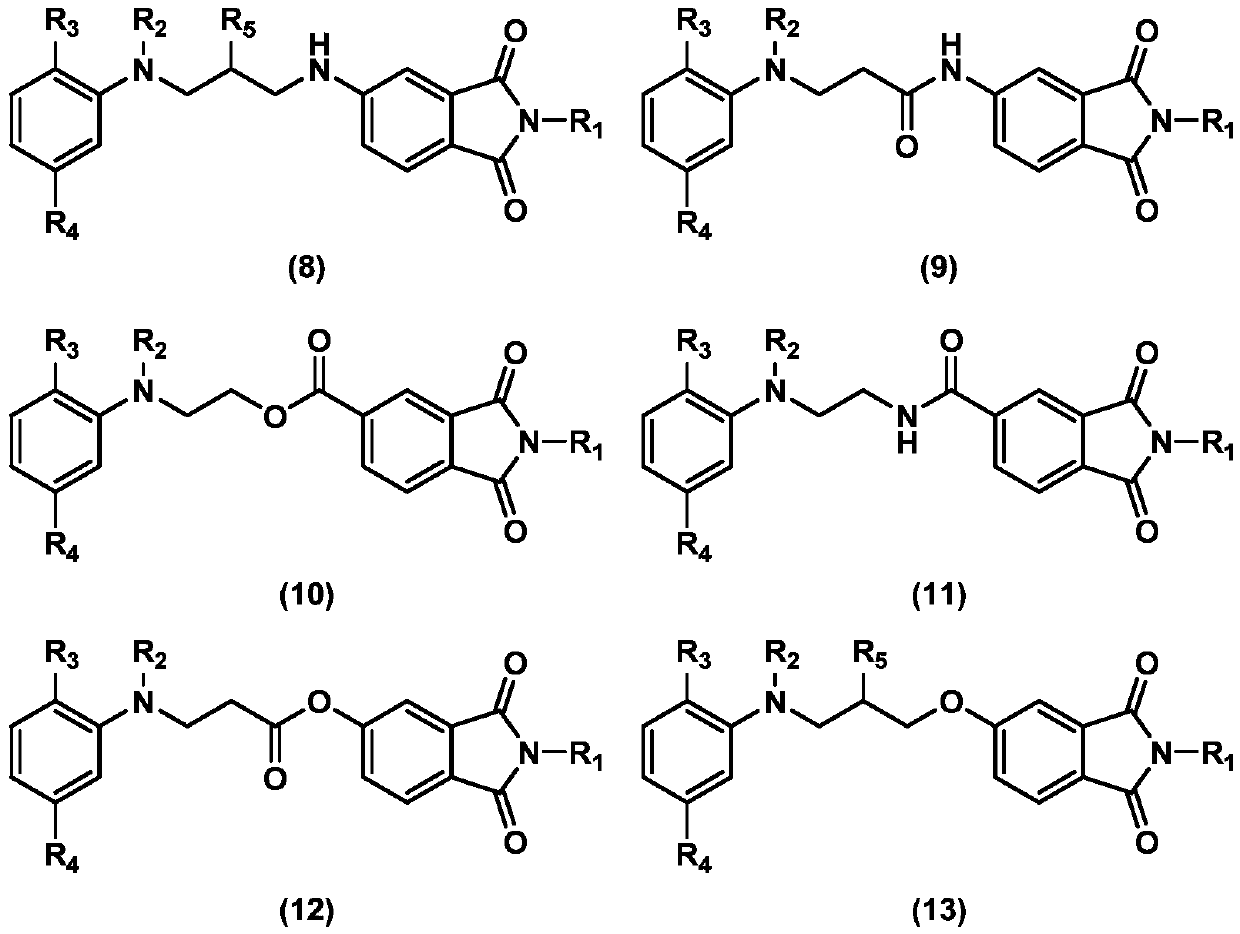
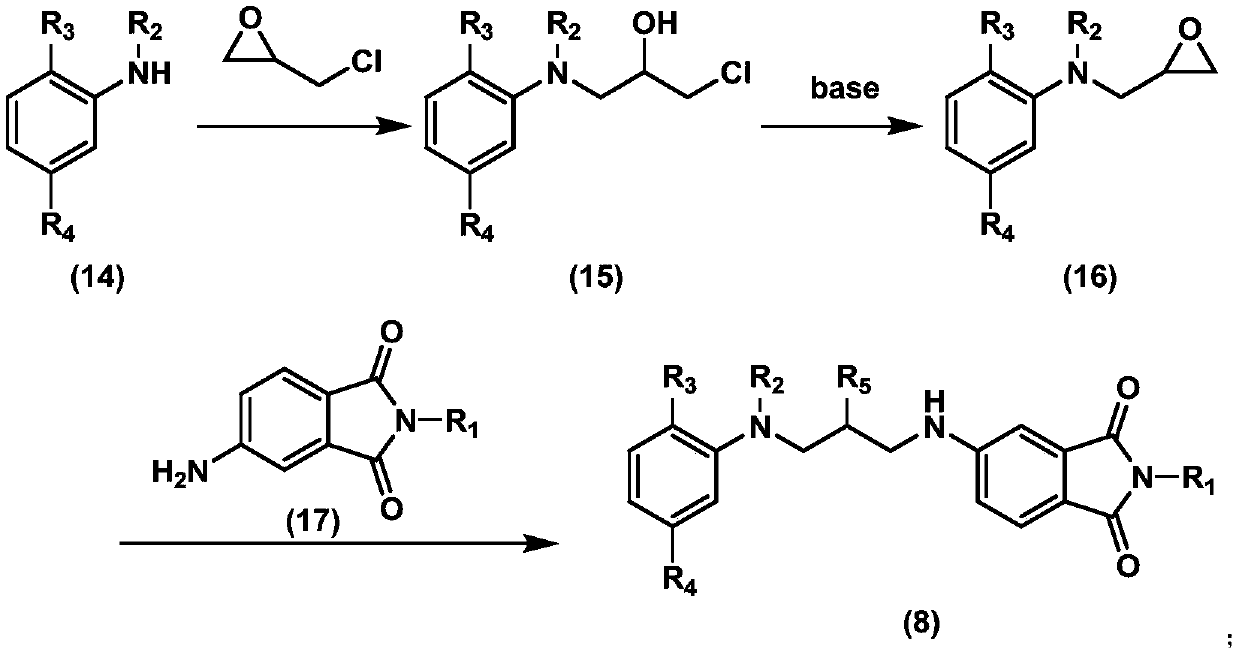
Coupling component containing phthalimide structure, and preparation method and application of coupling component
A technology of phthalimide and coupling components, applied in the field of coupling components and their preparation, can solve the problems of poor washing fastness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 10.8g of N-methylaniline, 13.9g of epichlorohydrin, and 20mL of acetonitrile as a solvent into a 100mL three-necked reaction flask with a stirrer and a thermometer, and add another 0.3g of zinc chloride catalyst; while stirring, heat to reflux and keep warm Reaction for 5 hours; liquid phase monitoring raw material N-methylaniline reaction is complete; cooling to room temperature, slowly adding 14g of 50% potassium hydroxide solution; adding 20 mL of water, stirring at room temperature for 4 hours; liquid phase monitoring cyclization is complete; Add 30mL of water and 30mL of ethyl acetate to the reaction solution, extract and separate the layers, wash the organic layer with 30mL×3 water, and dry with anhydrous magnesium sulfate; distill off the solvent to obtain 15.1g of oily N-(2,3-epoxypropyl) -N-methylaniline, see formula (16-1), yield 92.6%, liquid chromatography purity 95%, LC-MS (ESI) positive ion mode: m / z164, [M+H] + ,m / z186,[M+Na] + ,m / z202[M+K] + .
[0065] ...
Embodiment 2
[0071] Add 22.7g 4-amino-N-butylphthalimide, 13.9g epichlorohydrin, and 50mL acetonitrile as solvent into a 150mL three-necked reaction flask with thermometer and stirring, and add 1.40g zinc chloride as a catalyst ; Under stirring, the temperature is raised to reflux, and the temperature is kept for 8 hours; the liquid phase monitors the completion of the raw material reaction; the ice-water bath is cooled to 0-5 ℃, and 27g 30% potassium hydroxide solution is slowly added; the reaction is stirred at 0-5 ℃ for 5 hours; The monitoring and environmentalization is completed;
[0072] Post-treatment: add 30mL water and 30mL dichloromethane to the reaction solution, extract and separate the layers, wash the organic layer with 30mL×3 water, and dry with anhydrous magnesium sulfate; distill off the solvent to obtain a thick substance, crystallize with ethanol to obtain 23.4g of light yellow solid Namely 4-[N-(2',3'-epoxypropyl)]amino-N-butylphthalimide, see formula (19-1), yield 85%; li...
Embodiment 3
[0076] 13.8g 4-[N-(2',3'-epoxypropyl)]-amino-N-butylphthalimide, see formula (19-1), 9.0g m-acetamido-N- Ethylaniline and 50mL acetonitrile were added to a 100mL reaction flask with a thermometer and stirring, and another 1.0g p-toluenesulfonic acid was added; the reaction was kept under reflux and stirred for 10 hours, and the reaction progress was monitored in the liquid phase. The solvent is distilled off to obtain 18.4g of thick material that is the coupling component, see formula (8-3), the yield is 81.4%, the liquid phase purity is 92%, LC-MS (ESI) positive ion mode: m / z453, [M +H] + ,m / z475,[M+Na] + ,m / z491[M+K] + .
[0077]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com