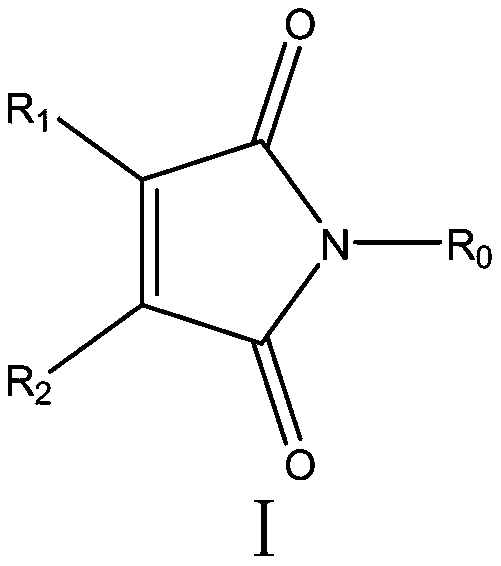
Application of maleimide compound as chitin synthase inhibitor
A technology of maleimide and chitin synthase, which can be used in application, biocide, animal repellent, etc., can solve problems such as economic losses, achieve less toxic and side effects on human body, and cheap and easy-to-obtain raw materials , the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
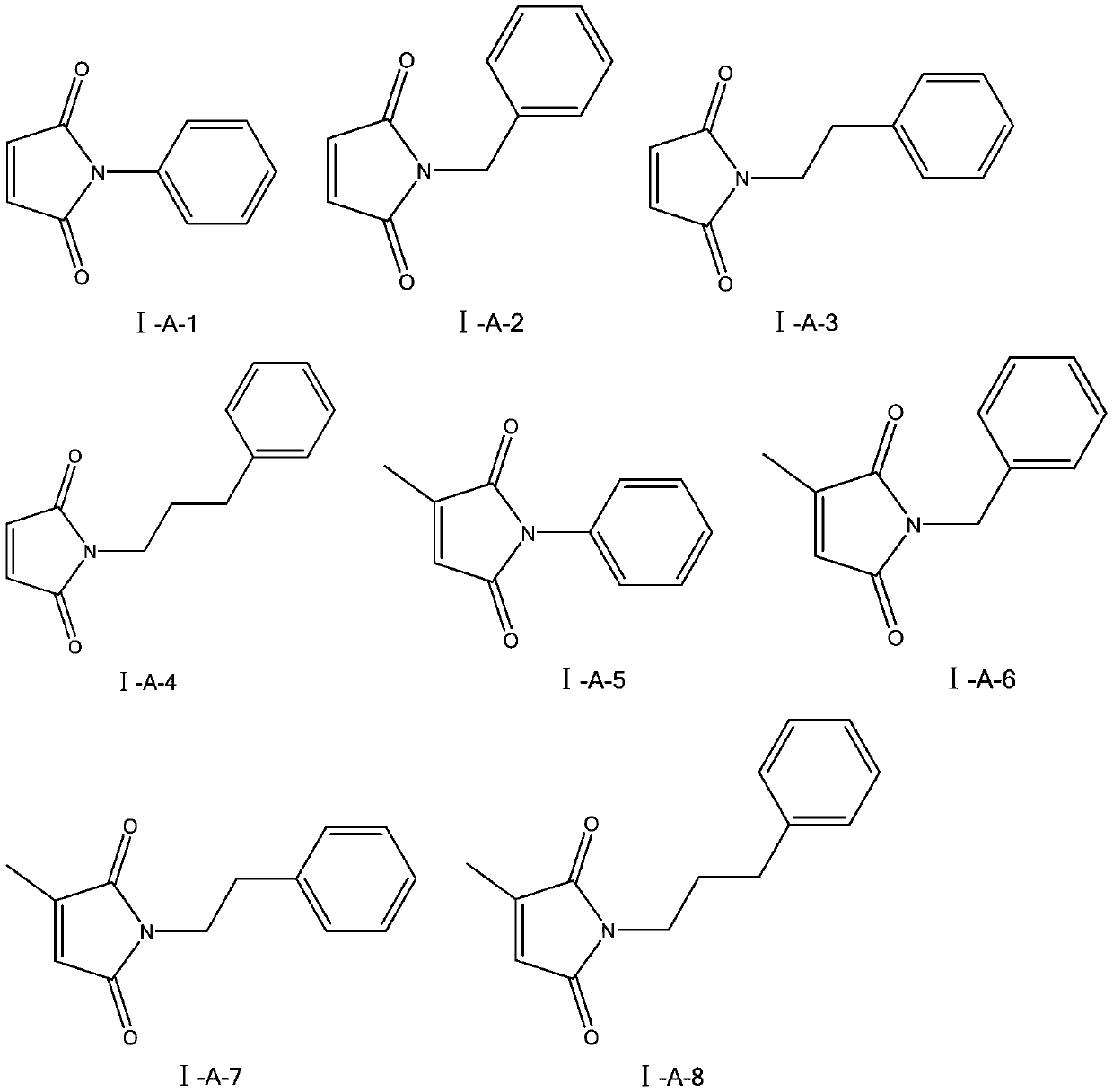
[0025] Embodiment 1: the synthesis of N-phenyl-maleimide (I-A-1):
[0026]
[0027] Weigh 0.98g of maleic anhydride (II-1) and 0.93g of aniline (III-1) into a three-necked round-bottomed flask, add 15mL of acetone and react at room temperature for 1 hour to generate the corresponding maleamic acid. Add 5 mL of acetic anhydride and 100 mg of sodium acetate to the generated maleamic acid, then heat at reflux for 2-3 h. After the reaction finished, the reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a concentrated solution, which was separated by silica gel column chromatography (eluent V 石油醚 :V 乙酸乙酯 =8:1), and the target liquid was collected, the solvent was removed by vacuum rotation, and the target product N-phenyl-maleimide (I-A-1) was obtained by vacuum drying at 60°C.
[0028] Yield 78.6%, Purity 96.7%. 1 H NMR (500MHz, CDCl3) δ7.38-7.35(m, 2H), 7.35-7.31(m, 2H), 7.29(dq, J=3.9, 1.7Hz, 1H), 6.72(d, J...
Embodiment 2
[0029] Embodiment 2: the synthesis of N-benzyl-maleimide (I-A-2):
[0030]
[0031] In Example 1, the raw material aniline (Ⅲ-1) was changed to benzylamine (Ⅲ-2), and other operations were the same as in Example 1, and the feed ratio remained unchanged.
[0032] Yield 73.3%, Purity 97.9%. 1 H NMR (500MHz CDCl 3 )δ7.38-7.35(m,2H),7.35-7.31(m,2H),7.29(dq, J=3.9,1.7Hz,1H),6.72(d,J=5.0Hz,2H),4.69(s ,2H).HRMS[M+H] + :188.1985.
Embodiment 3
[0033] Embodiment 3: the synthesis of N-phenethyl-maleimide (I-A-3):
[0034]
[0035] In Example 1, the raw material aniline (III-1) was changed to phenethylamine (III-3), and other operations were the same as in Example 1, and the feed ratio remained unchanged.
[0036] Yield 77.2%, Purity 95.3%. 1 H NMR (500MHz, CDCl 3 )δ7.50-7.46(m,2H),7.29-7.24(m,2H),7.24-7.18(m,2H),7.16(s,1H),3.69-3.65(m,2H),2.86-2.82( m,2H).HRMS[M+H] + :202.0785.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com