Preparation method of normal-temperature bromoethyl cyanobiphenyl based on dibromohydantoin
A technology of bromosartan biphenyl and dibromohydantoin, which is applied in the field of medicine and chemical industry, can solve the problems of inability to apply continuous and semi-continuous automatic production lines, increase the pressure of environmental protection treatment, increase the pressure of waste water treatment, etc. Excellent atomic economy, shortened reaction time, and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
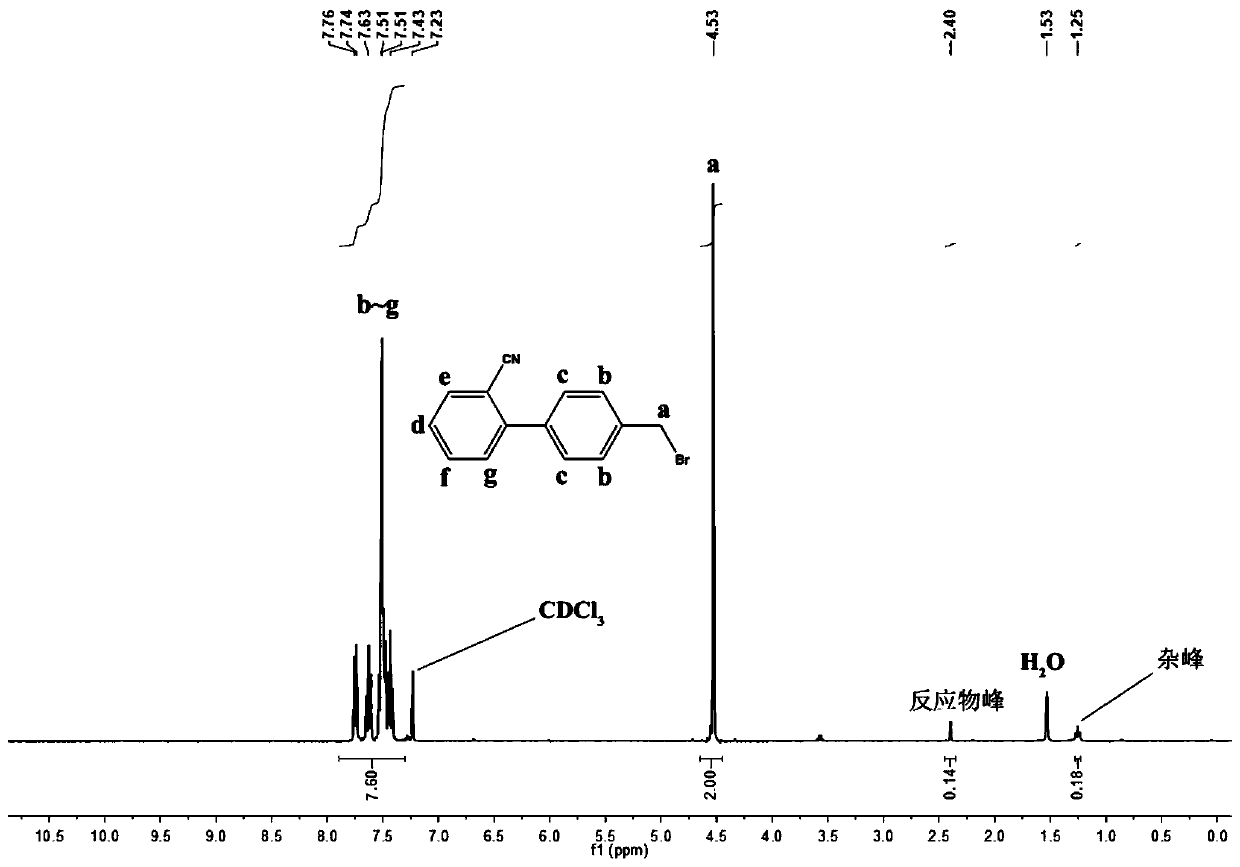
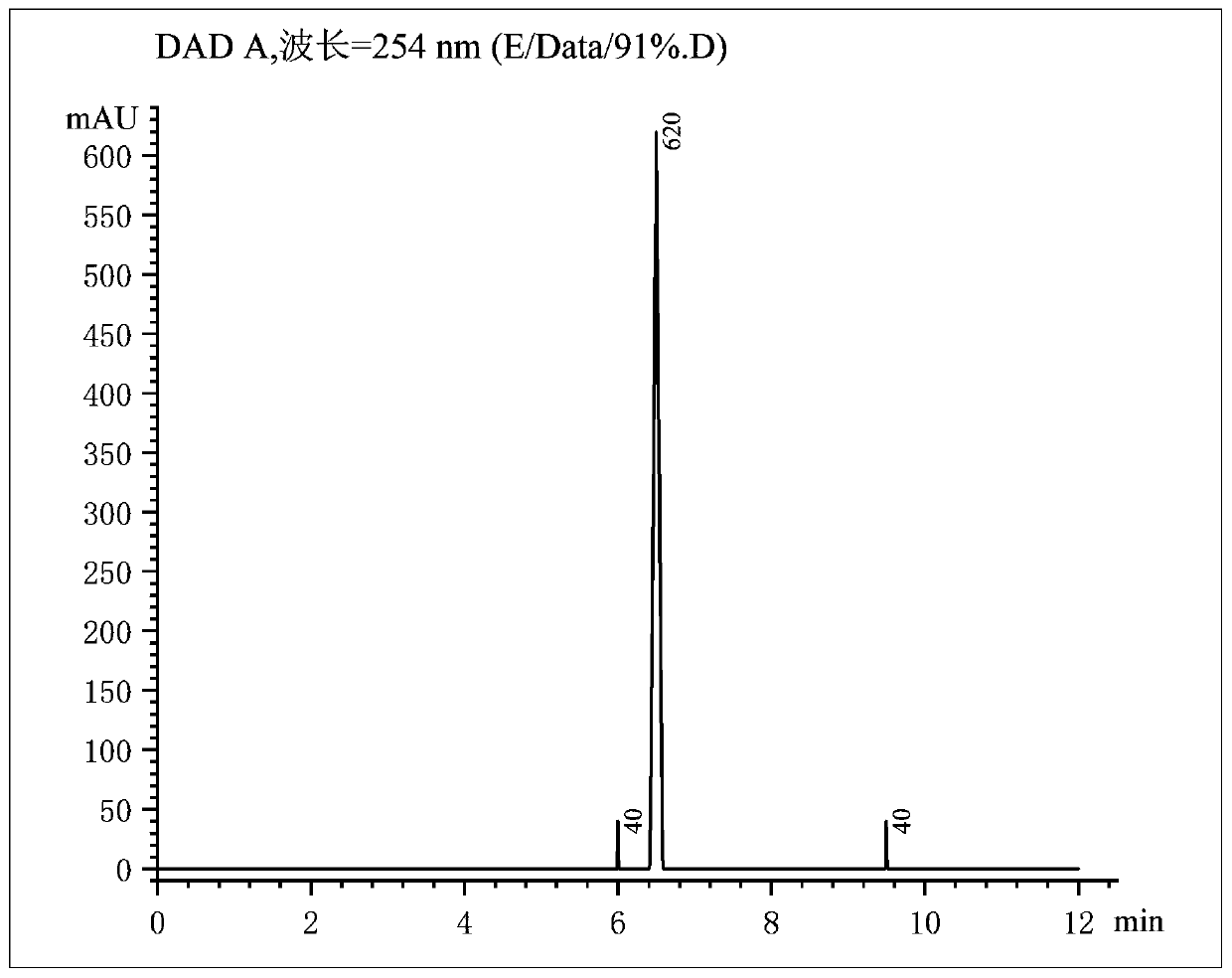
[0038] A method for preparing bromosartan biphenyl at room temperature based on dibromohydantoin, 10g of 2-cyano-4-methylbiphenyl is dissolved in 30mL of acetone, and put into syringe A tube; 9g of dibromohydantoin Dissolve in 30mL acetone and put it into syringe B tube. Install the two syringes A and B on the flow syringe pump, assemble them, and set the flow rate of both tubes A and B to 1.5mL / min. Turn on the constant temperature water bath device, heat to 55°C, keep the temperature, turn on the 365nm 4000K LED light for 30 minutes, and turn on the flow reactor at the same time. After completion of the reaction, add 15 g of sodium bisulfite to the received product to quench, separate the liquids, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, filter with suction, and put the filter cake in an oven to dry to obtain pure product The product 2-cyano-4-bromomethylbiphenyl has a purity of 91% and a yield of 90%.
Embodiment 2
[0040] A method for preparing bromosartan biphenyl at room temperature based on dibromohydantoin, 10g of 2-cyano-4-methylbiphenyl is dissolved in 30mL of acetone, and put into syringe A tube; 9g of dibromohydantoin Dissolve in 30mL acetone and put it into syringe B tube. Install the two syringes A and B on the flow syringe pump, assemble them, and set the flow rate of both tubes A and B to 1.5mL / min. Turn on the constant temperature water bath device, heat to 30°C, keep the temperature, turn on the 385nm 4000K LED light for 30 minutes, and turn on the flow reactor at the same time. After completion of the reaction, add 15 g of sodium bisulfite to the received product to quench, separate the liquids, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, filter with suction, and put the filter cake in an oven to dry to obtain pure product The product 2-cyano-4-bromomethylbiphenyl has a purity of 90% and a yield of 93%.
Embodiment 3
[0042] A method for preparing bromosartan biphenyl at room temperature based on dibromohydantoin, 10g of 2-cyano-4-methylbiphenyl is dissolved in 30mL of acetone, and put into syringe A tube; 9g of dibromohydantoin Dissolve in 30mL acetone and put it into syringe B tube. Install the two syringes A and B on the flow syringe pump, assemble them, and set the flow rate of both tubes A and B to 1.5mL / min. Turn on the constant temperature water bath device, heat to 60°C, keep the temperature, turn on the 4000K LED light of 405nm for 30 minutes, and turn on the flow reactor at the same time. After completion of the reaction, add 15 g of sodium bisulfite to the received product to quench, separate the liquids, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, filter with suction, and put the filter cake in an oven to dry to obtain pure product The product 2-cyano-4-bromomethylbiphenyl has a purity of 91% and a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com