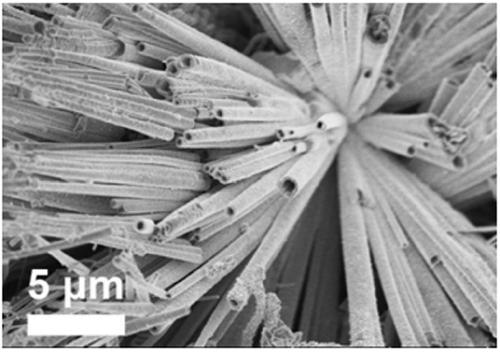
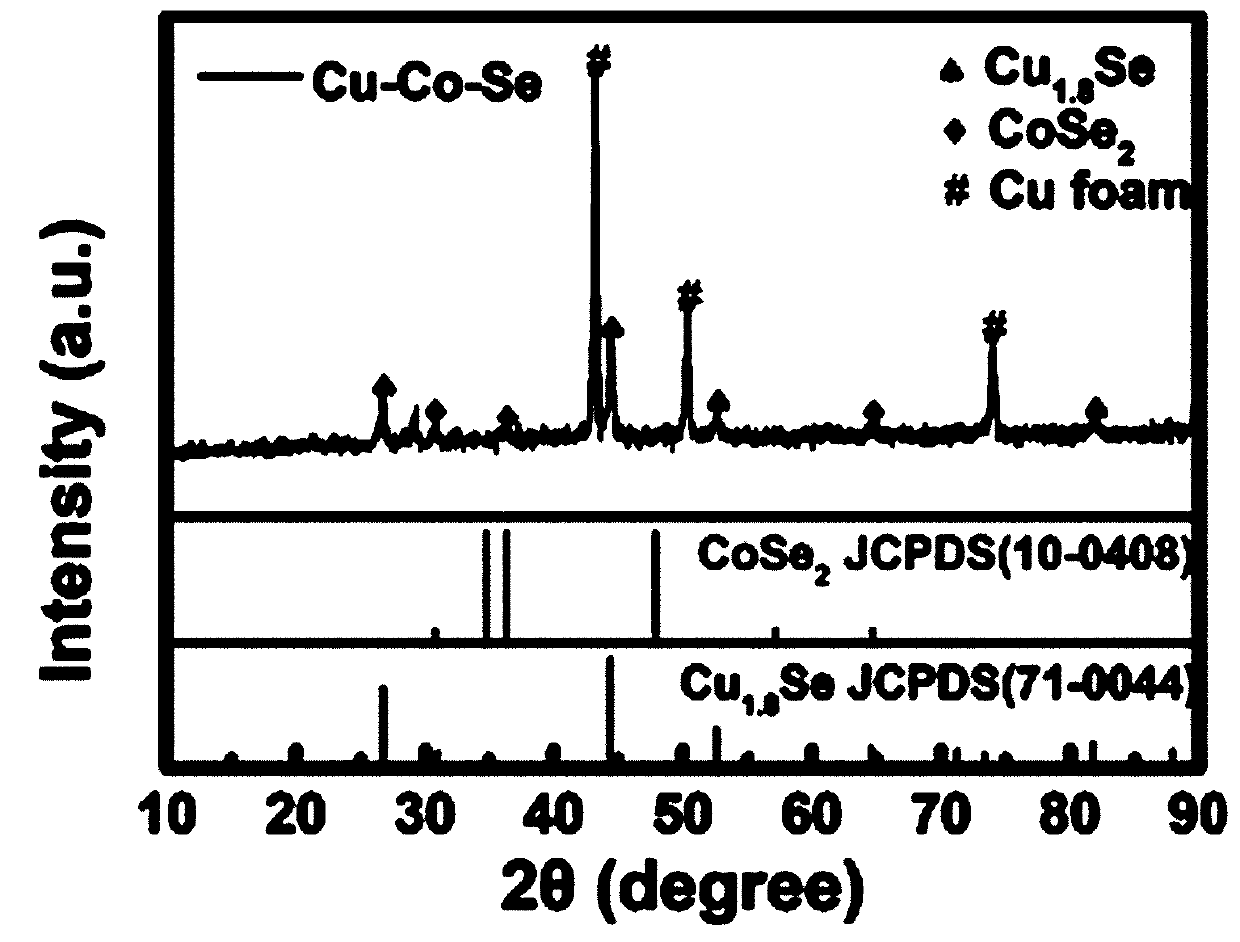
Self-supporting high-density copper-cobalt-selenium nanotube electrode and preparation method thereof
A high-density, selenium nanotechnology, applied in the direction of electrodes, nanotechnology, nanotechnology, etc., can solve the problems of lowering the reaction energy barrier, low storage, high price, etc., and achieve the improvement of catalytic activity, accelerated transmission process, and large specific surface area. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of a self-supporting high-density copper-cobalt-selenium nanotube electrode is carried out according to the following steps:
[0026] Step 1: Sonicate the copper foam (1.0 cm×4.0 cm×1.5 mm) with hydrochloric acid (3.0 M HCl), acetone, absolute ethanol, and ultrapure water for several minutes in sequence, and dry it for later use.
[0027] Step 2: Stir 50 mL of a mixed solution of 0.08 M cobalt chloride, 0.04 M copper chloride and 0.16 M urea evenly, put it into a hydrothermal reaction kettle together with foamed copper, and then put the reaction kettle into a hydrothermal tank for Hydrothermal reaction, the reaction time is 12 h, and the reaction temperature is 120 °C.
[0028] Step 3: The sample Cu-Co-O obtained after the hydrothermal reaction was repeatedly washed several times with ultrapure water and alcohol to remove impurities and precipitates on the surface, and then dried in a vacuum drying oven for 8 hours.
[0029] Step 4: Stir 3 mL of a ...
Embodiment 2
[0034] A preparation method of a self-supporting high-density copper-cobalt-selenium nanotube electrode is carried out according to the following steps:
[0035] Step 1: Clean the copper foam (1.0 cm × 4.0 cm × 1.5 mm) with hydrochloric acid (3.0 M HCl), acetone, absolute ethanol, and ultrapure water for several minutes, and dry it for later use.
[0036] Step 2: Stir 50 mL of a mixed solution of 0.04 M cobalt chloride, 0.02 M copper chloride and 0.12 M urea evenly, put it into a hydrothermal reaction kettle together with foamed copper, and then put the reaction kettle into a hydrothermal tank for Hydrothermal reaction, the reaction time is 8 h, the reaction temperature is 100 °C.
[0037]Step 3: The sample Cu-Co-O obtained after the hydrothermal reaction was repeatedly washed several times with ultrapure water and alcohol to remove impurities and precipitates on the surface, and dried in a vacuum drying oven for 8 hours.
[0038] Step 4: Stir 3 mL of a mixed solution contain...
Embodiment 3
[0042] A preparation method of a self-supporting high-density copper-cobalt-selenium nanotube electrolysis water electrode is carried out according to the following steps:
[0043] Step 1: Sonicate foamed copper (1.0 cm×4.0 cm×1.5 mm) with hydrochloric acid (3.0 M HCl), acetone, absolute ethanol, and ultrapure water for several minutes in sequence, and dry it for later use.
[0044] Step 2: Stir 50 mL of a mixed solution of 0.05 M cobalt chloride, 0.1 M copper chloride and 0.18 M urea evenly, put it into a hydrothermal reaction kettle together with foamed copper, and then put it into a hydrothermal tank for hydrothermal reaction , the reaction time is 14h, and the reaction temperature is 150°C.
[0045] Step 3: The sample Cu-Co-O after the hydrothermal reaction was repeatedly washed several times with ultrapure water and alcohol to remove impurities and precipitates on the surface, and then dried in a vacuum drying oven for 8 hours.
[0046] Step 4: Stir 3 mL of a mixed solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com