Method of detecting membrane protein of extracellular vesicles
A membrane protein and cell technology, which can be used in measurement devices, biological tests, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] The preparation of embodiment 1 antibody chip
[0087] The positive control biotin-labeled IgG antibody concentration was 0.04 μg / ml, 0.2 μg / ml, 1 μg / ml, 5 μg / ml, and the negative control was PBS solution. The specific antibody concentration against EVs membrane protein was 200μg / ml, dissolved in PBS containing 5% glycerol. The manufacturer of the specific antibody is Sino Biological Inc. The details of the name and article number of the specific antibody are shown in Table 1.
[0088] Table 1 Name and catalog number of specific antibody
[0089]
[0090]
[0091]
[0092]
[0093]
[0094]
[0095]
[0096]
[0097] The solid phase carrier adopts NEXTERION epoxy-coated glass slides from SCHOTT Company of Germany. The above-mentioned antibodies are spotted on the coated glass slides according to a certain arrangement using an automatic spotting instrument, and two replicates are set for each antibody spot.
Embodiment 2
[0098] Embodiment 2 sample pretreatment
[0099]This example is for the preliminary separation of EVs from the CCC-HEL-1 cell culture supernatant: collect the CCC-HEL-1 cell culture supernatant, centrifuge at 300g for 10min, collect the supernatant, and remove the cells; then centrifuge at 3000g for 10min, and collect the supernatant , remove cell debris; then centrifuge at 10000g for 30min, collect supernatant to remove subcellular components; then concentrate with 100KD ultrafiltration centrifuge tube, wash with PBS 3 times, and concentrate to about 100 times in final volume.
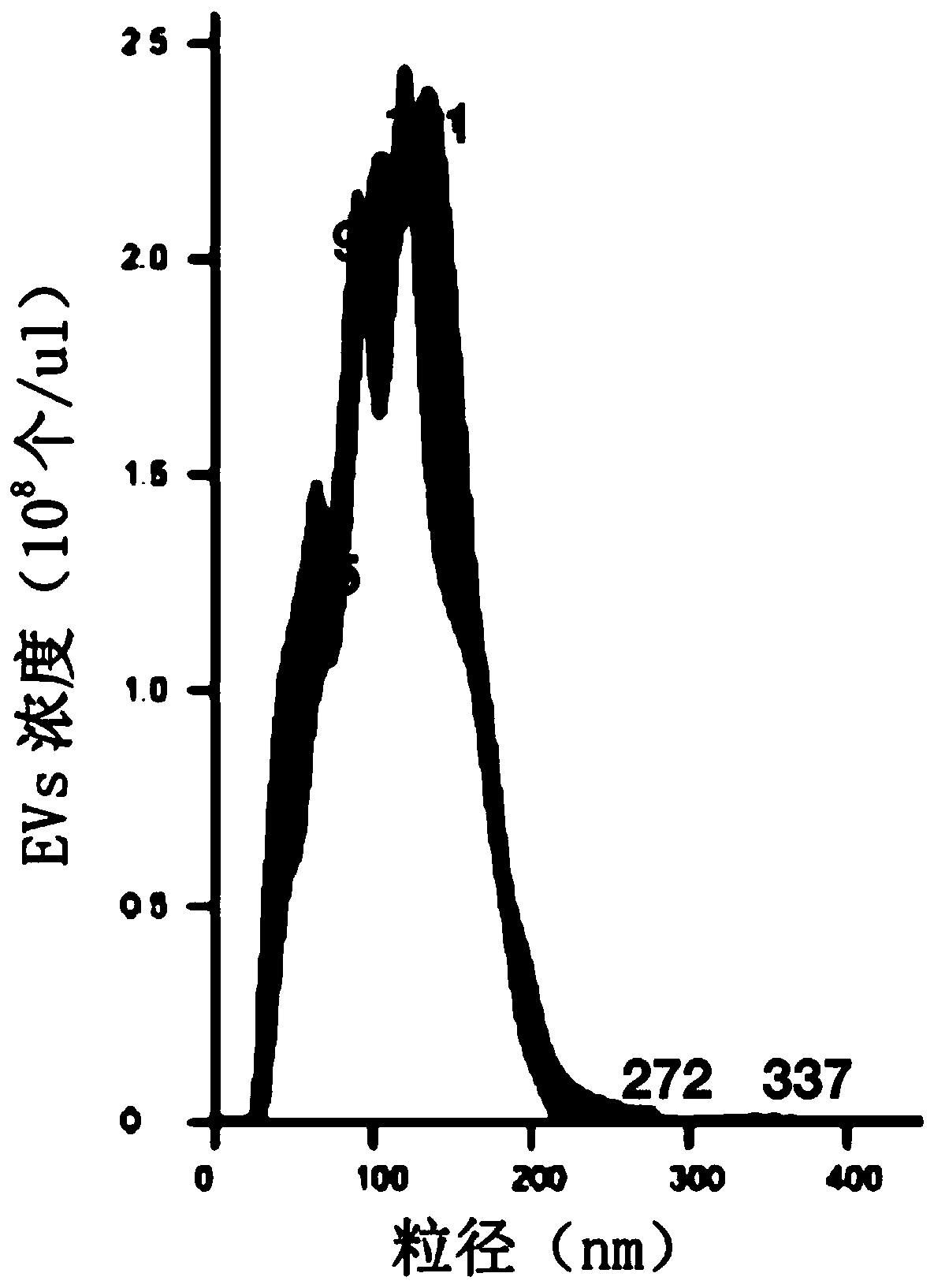
[0100] Then, the particle size and concentration analysis of the EVs obtained from the preliminary separation was carried out using the Malvern nanoparticle tracking analyzer NanoSight LM14. Test results such as figure 1 As shown, the concentration of EVs in the pretreated samples was 2.3×10 8 particles / μl, the particle size is mainly distributed around 150nm.
Embodiment 3
[0101] Example 3 Antibody Chip Separation of EVs and Analysis of EVs Membrane Protein Composition
[0102] Add the pre-treated samples to the antibody chip to isolate EVs. The specific method is as follows:
[0103] (1) Adding samples: the concentration of pretreated EVs added to the antibody chip was 10 5 -10 8 per μl, the volume added was 100 μl. Incubate at room temperature for 2 h on a horizontal shaker at a low speed (60 rpm / min). Then transfer to 4°C and continue to incubate for 18h.
[0104] (2) Washing: Aspirate the sample from the sample pool, add 100 μl of PBS solution containing 0.2% Tween20 to wash 3 times, 5 min each time.
[0105] (3) Detection: Add a mixture of detection antibodies (biotin-labeled antibodies against EVs membrane protein markers CD9, CD63, and CD81), and incubate at room temperature for 2 hours on a horizontal shaker at a low speed (60 rpm / min). Aspirate the biotin-labeled antibody mixture from the sample pool, and repeat the washing proces...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com