Preparation method of 4'-bromomethyl-2-cyanobiphenyl dissolved in 1,2-dichloroethane
A technology of bromosartan biphenyl and dichloroethane, which is applied in the field of medicine and chemical industry, can solve the problems of uneven distribution of reactants, increased waste water treatment pressure, high raw material cost, etc., achieves excellent atom economy, and reduces treatment costs Difficulty and the effect of reducing security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
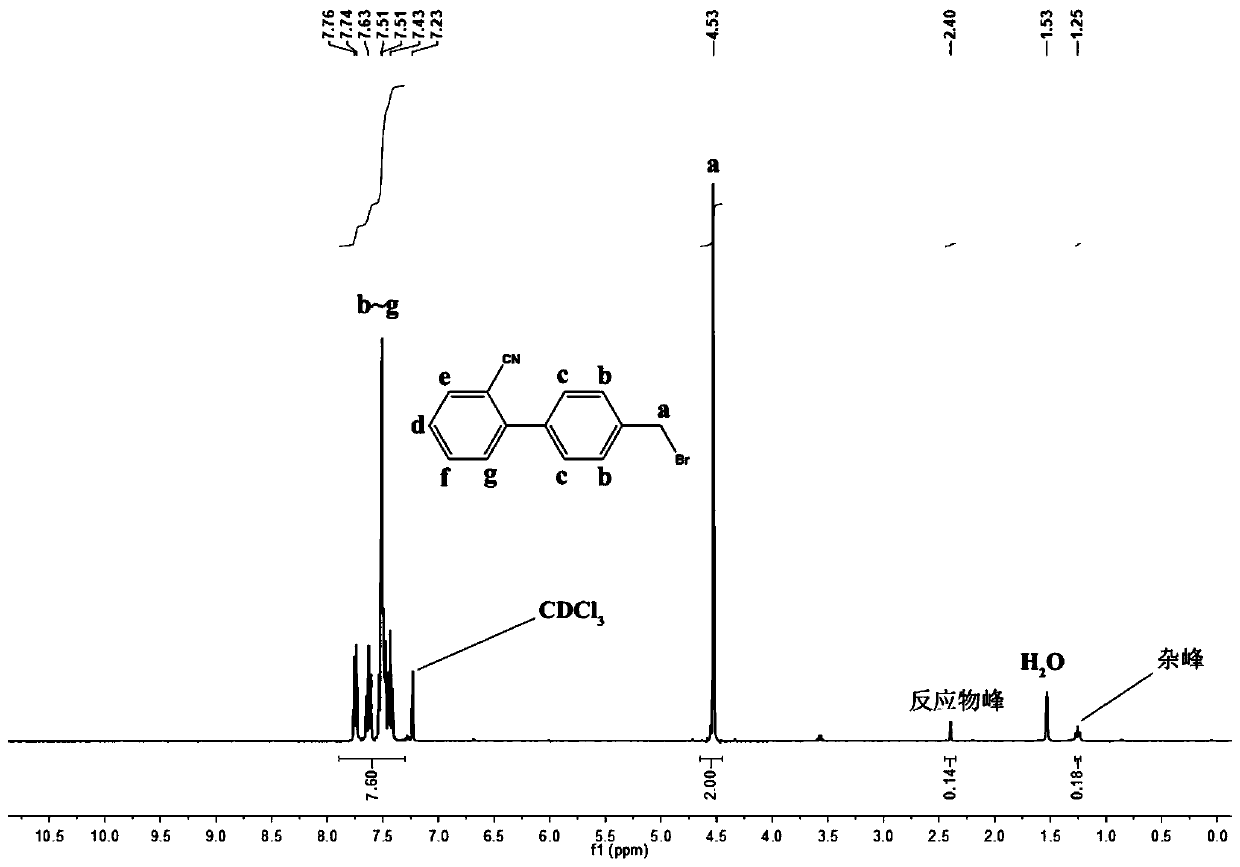
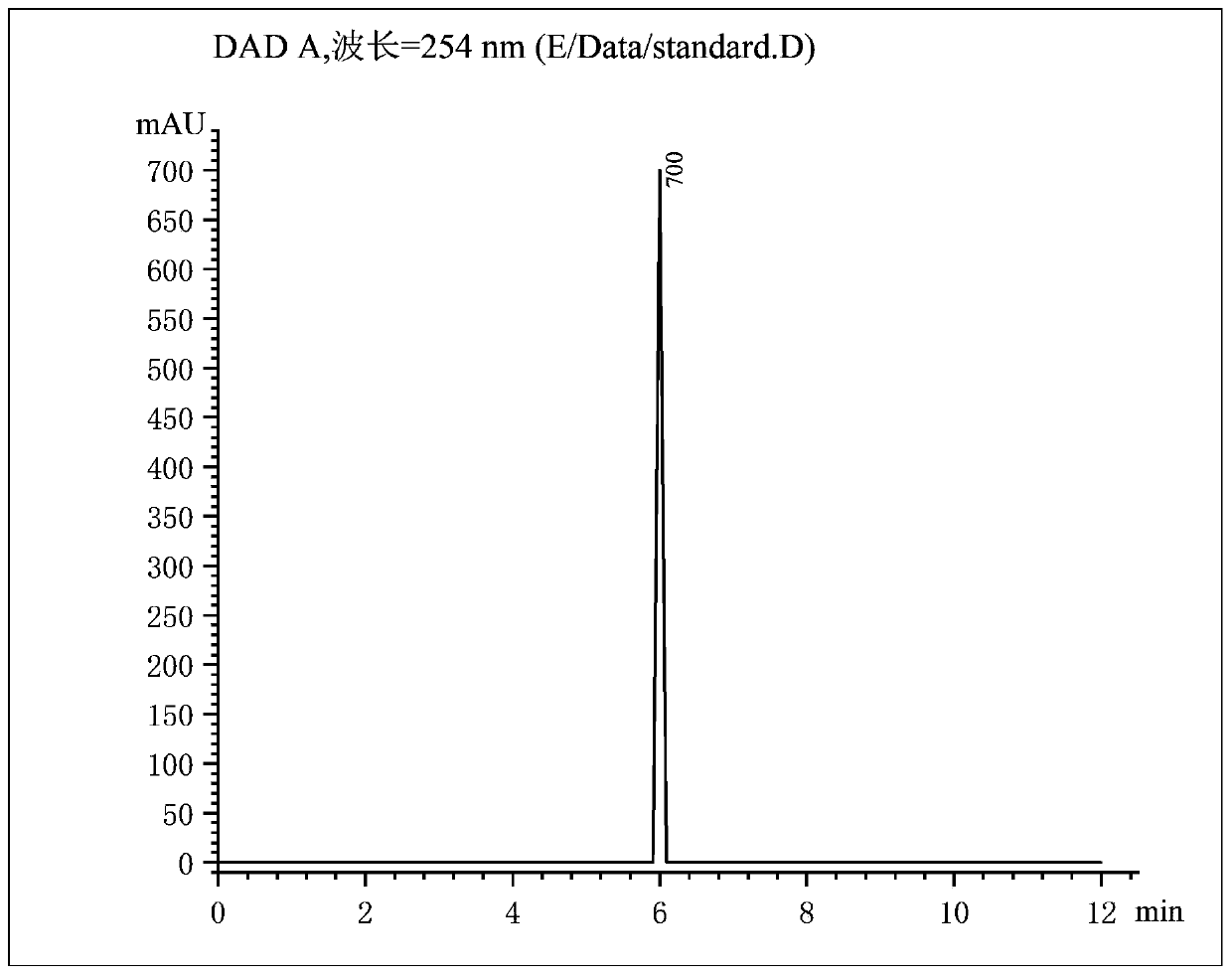
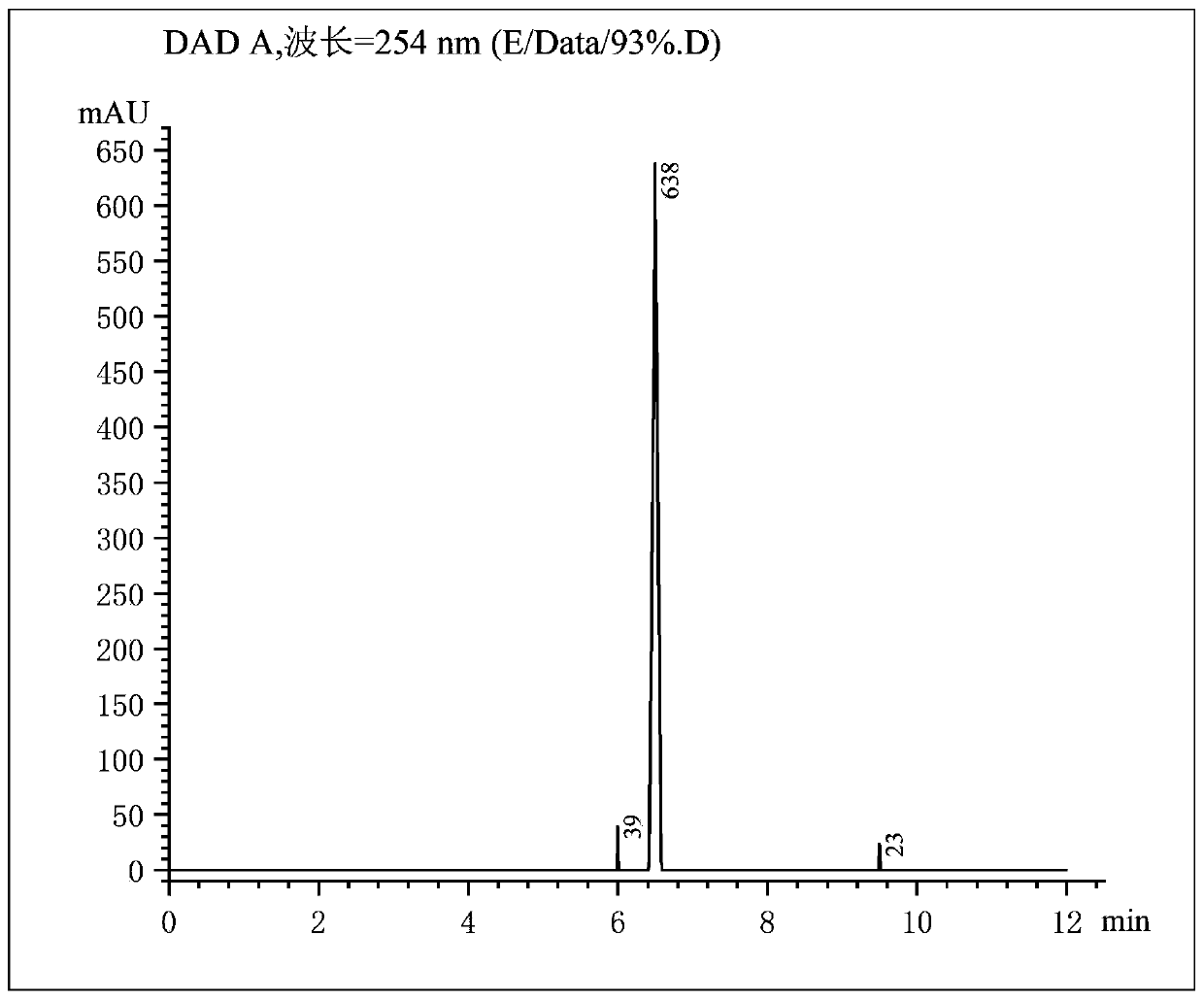
[0037]A method for preparing bromosartan biphenyl dissolved in 1,2-dichloroethane, dissolving 10g of 2-cyano-4-methyl biphenyl in 30mL of 1,2-dichloroethane, and loading it into a syringe In tube A; dissolve 9 g of N-bromosuccinimide in 30 mL of 1,2-dichloroethane and put it into tube B of the syringe. Install the two syringes A and B on the flow syringe pump, and set the flow rates of the two tubes A and B to be 1.5 mL / min. Turn on the constant temperature water bath device, heat to 70°C, keep the temperature, turn on the 4000K LED light at 365nm for 30min, and turn on the flow reactor at the same time. After the reaction is completed, add 15 g of sodium bisulfite to the received product to quench, separate the liquid, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, suction filtration, and put the filter cake in an oven to dry to obtain a pure product. The product, 2-cyano-4'-bromomethylbiphenyl, has a purity of 93% a...
Embodiment 2
[0039] A method for preparing bromosartan biphenyl dissolved in 1,2-dichloroethane, dissolving 11g of 2-cyano-4-methylbiphenyl in 30mL of 1,2-dichloroethane and filling it into a syringe In tube A; dissolve 7 mL of bromine in 30 mL of 1,2-dichloroethane and put it into tube B of the syringe. Install the two syringes A and B on the flow syringe pump, and set the flow rates of the two tubes A and B to be 1.5 mL / min. Turn on the constant temperature water bath device, heat to 70°C, keep the temperature, turn on the 4000K LED light at 365nm for 30min, and turn on the flow reactor at the same time. After the reaction is completed, add 15 g of sodium bisulfite to the received product to quench, separate the liquid, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, suction filtration, and put the filter cake in an oven to dry to obtain a pure product. The product, 2-cyano-4'-bromomethylbiphenyl, has a purity of 92% and a yield ...
Embodiment 3
[0041] A method for preparing bromosartan biphenyl dissolved in 1,2-dichloroethane, dissolving 11g of 2-cyano-4-methylbiphenyl in 30mL of 1,2-dichloroethane and filling it into a syringe In tube A; dissolve 4 g of sodium bromide and 3 g of sodium bromate in 30 mL of 1,2-dichloroethane, and put them into tube B of the syringe. Install the two syringes A and B on the flow syringe pump, and set the flow rates of the two tubes A and B to be 1.5 mL / min. Turn on the constant temperature water bath device, heat to 70°C, keep the temperature, turn on the 4000K LED light at 365nm for 30min, and turn on the flow reactor at the same time. After the reaction is completed, add 15 g of sodium bisulfite to the received product to quench, separate the liquid, remove the solvent by rotary evaporation of the organic phase, add isopropyl ether for recrystallization, suction filtration, and put the filter cake in an oven to dry to obtain a pure product. The product, 2-cyano-4'-bromomethylbipheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com