Biomarker related to tumor immunotherapy effect and application of biomarker
A biomarker and immunotherapy technology, applied in the application field of predicting the effect of tumor immunotherapy, can solve problems such as adverse reactions, single biomarkers, test result sampling time and sampling site effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] (1) Preparation and immobilization of antigenic protein
[0160] The cDNA of tumor-associated antigen (TAA) was cloned into PET28(a) expression vector containing 6XHis tag. At the N-terminal or C-terminal of the antigen, streptavidin or the like (biotin-binding tag protein) is introduced. The obtained recombinant expression vector was transformed into Escherichia coli for expression. After the protein was expressed in the inclusion body, the protein was denatured with 6M guanidine hydrochloride, and refolded in vitro according to the standard method, and then Ni-NTA affinity was carried out through the 6XHis tag Column purification to obtain antigenic protein.
[0161] (2) Preparation of plasma
[0162] Venous blood was drawn in EDTA-treated or citric acid-treated blood collection tubes within one week to one day before immunotherapy. Then centrifuge at 1000-2000RCF at room temperature for 15 minutes; after centrifugation, gently transfer the supernatant to another c...
Embodiment 1
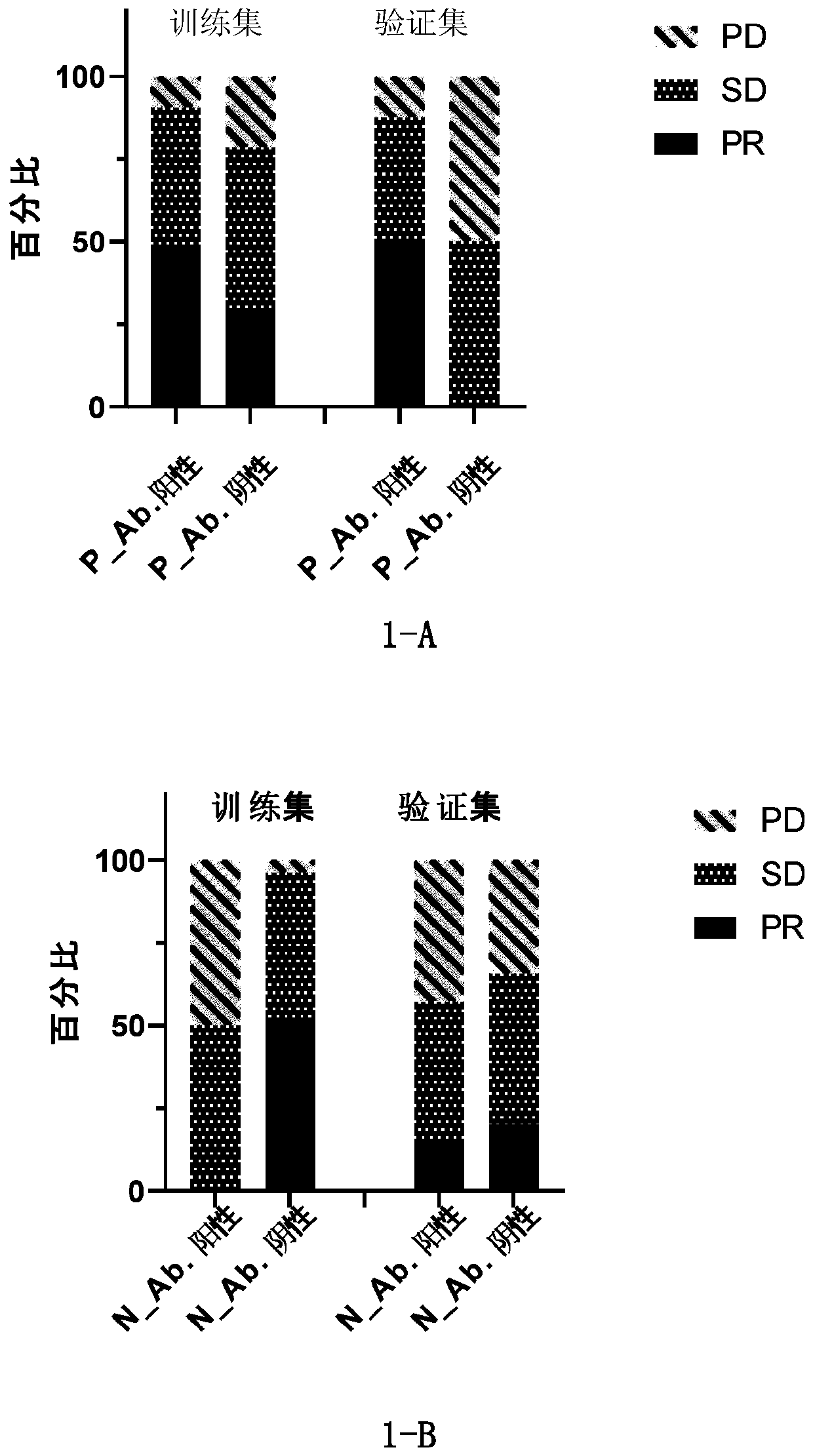
[0199] In order to find autoantibodies that can indicate the effect of immunotherapy, firstly, the plasma of 47 normal healthy people (healthy control group) and 47 patients diagnosed with lung cancer (lung cancer group) was used to detect whether there were autoantibodies against the purified antigenic protein in lung cancer patients . The healthy control group consisted of those who were not diagnosed with cancer in the past and present. The lung cancer group consisted of patients diagnosed with lung cancer, including 10 patients with small cell lung cancer, 12 patients with lung squamous cell carcinoma, 19 patients with lung adenocarcinoma, and 6 patients with other subtypes of lung cancer. Plasma was drawn before treatment. The experimental population information is shown in Table 1.
[0200] Table 1. Primary screening test crowd information
[0201]
[0202]
[0203] In order to compare the concentrations of autoantibodies corresponding to each antigen in the heal...
Embodiment 2
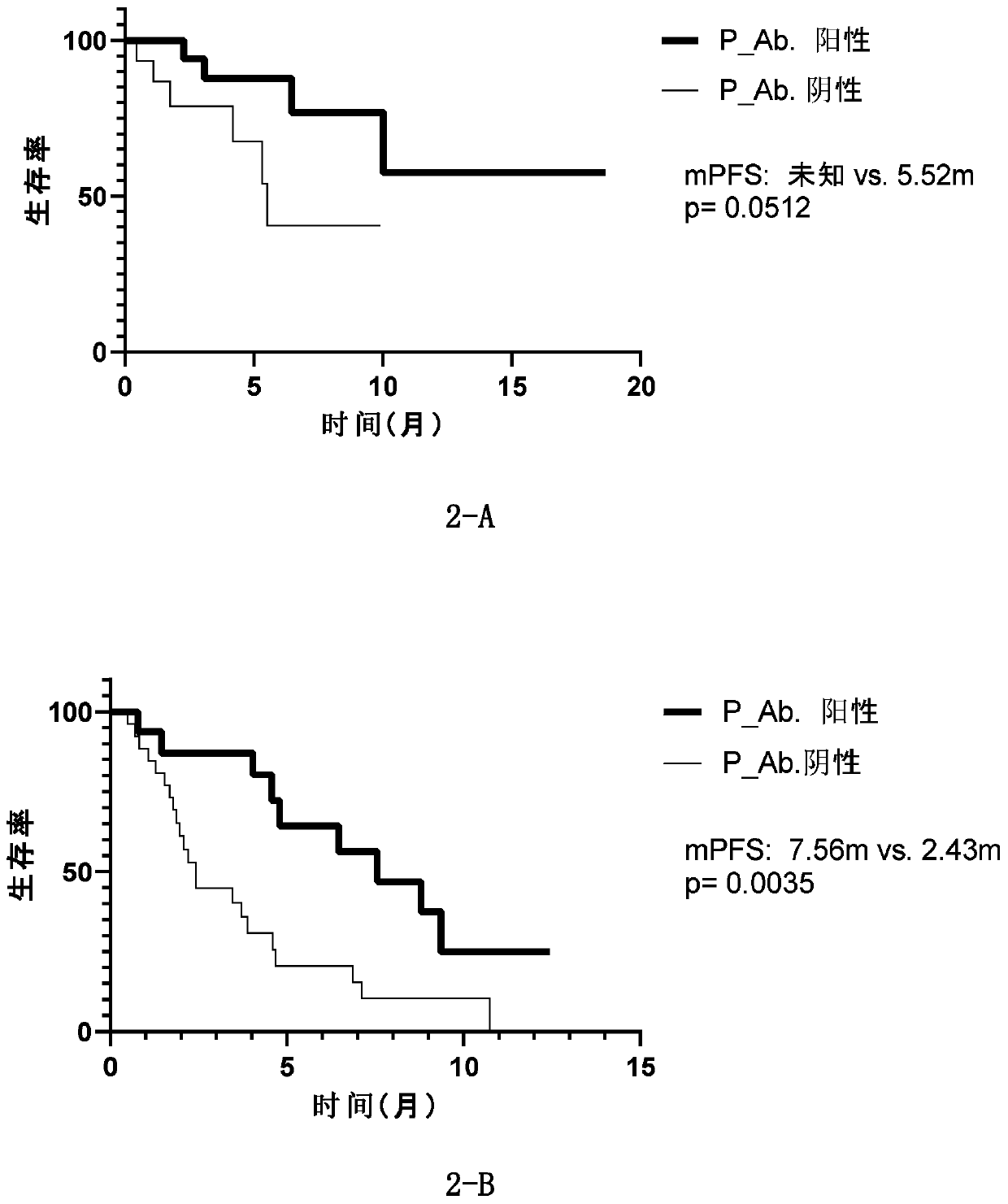
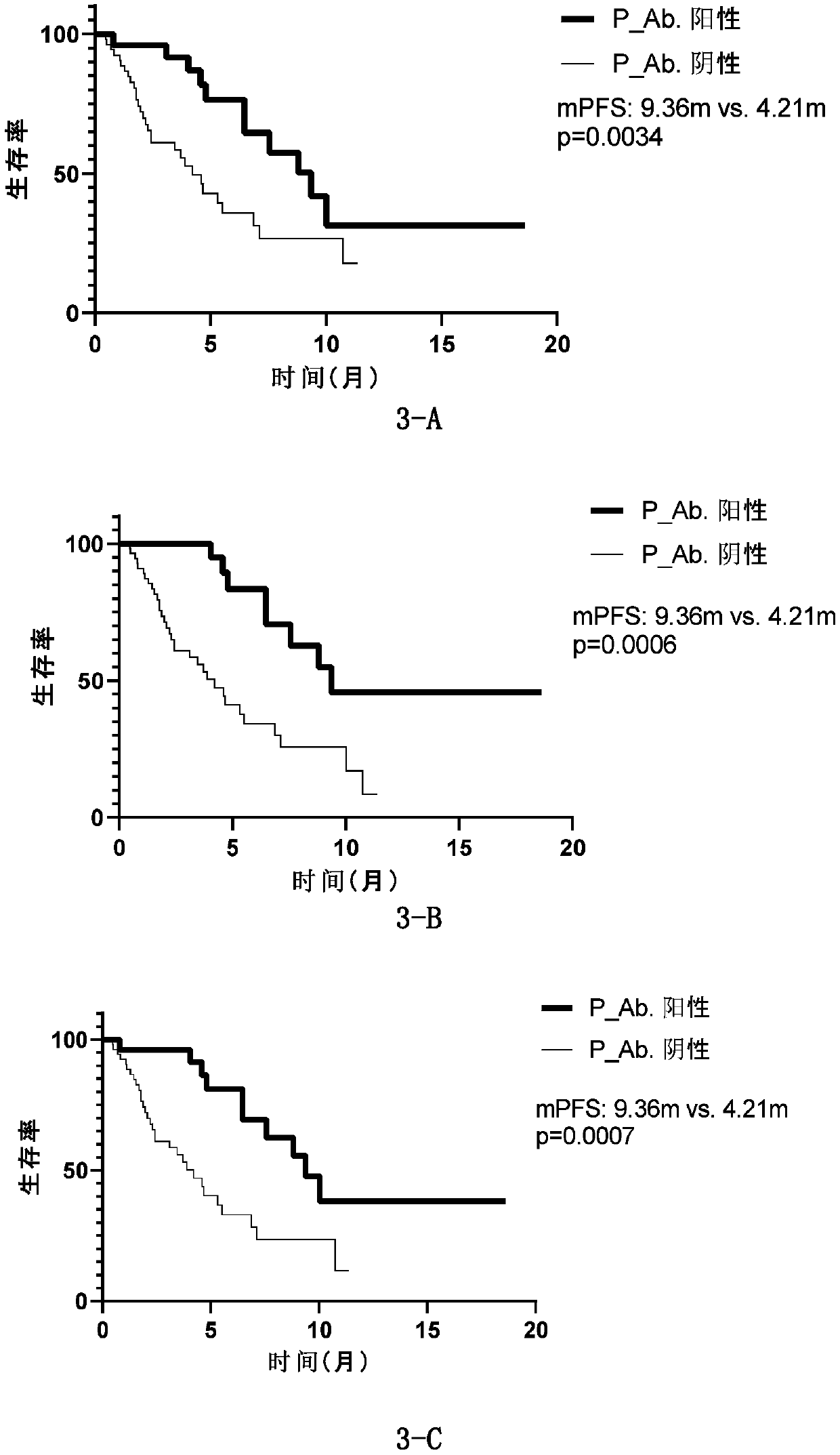
[0208] Autoantibodies are detected in the baseline (before treatment) plasma of immunotherapy patients (Table 3), and the antigenic proteins of the first three groups (sensitivity>5%) in Table 2 of Example 1 are used to screen for autoantibodies related to the curative effect of lung cancer patients. .
[0209] In order to screen immunotherapy predictive markers suitable for clinical application, this study included various scenarios of immunotherapy for lung cancer patients as much as possible. There are 38 lung cancer patients who use immunotherapy as first-line treatment, while 40 lung cancer patients have undergone one or more treatments such as chemotherapy and targeted therapy, and then choose immunotherapy (post-line treatment). In addition, the immunotherapy used by the enrolled patients included immune checkpoint inhibitors, including imported nivolumab, pembrolizumab, and domestic immune checkpoint inhibitors (Table 3). Whether it is first-line or later-line immunot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com